| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-07-13 19:45:23 UTC |
|---|
| Update Date | 2016-08-02 20:40:28 UTC |
|---|
| Lmdb | LMDB00237 |
|---|
| Secondary Accession Numbers | None |
|---|
| Metabolite Identification |
|---|
| Common Name | Malic acid |
|---|
| Description | Malic acid, also known as malate or aepfelsaeure, belongs to the class of organic compounds known as beta hydroxy acids and derivatives. Beta hydroxy acids and derivatives are compounds containing a carboxylic acid substituted with a hydroxyl group on the C3 carbon atom. Malic acid is an extremely weak basic (essentially neutral) compound (based on its pKa). Malic acid exists in all living organisms, ranging from bacteria to humans. Outside of the human body, Malic acid is found, on average, in the highest concentration within a few different foods, such as roselles, figs, and sea-buckthornberries and in a lower concentration in lettuces, mandarin orange (clementine, tangerine), and purslanes. Malic acid has also been detected, but not quantified in, several different foods, such as white lupines, rose hips, cow milks, chives, and parsley. This could make malic acid a potential biomarker for the consumption of these foods. Malic acid has been found to be a metabolite in Aspergillus (Hugo Vanden Bossche, D.W.R. Mackenzie and G. Cauwenbergh. Aspergillus and Aspergillosis, 1987). Malic Acid has been used in trials studying the treatment of Xerostomia, Depression, and Hypertension. Malic acid is a tart-tasting organic dicarboxylic acid that plays a role in many sour or tart foods. In its ionised form it is malate, an intermediate of the TCA cycle along with fumarate. It can also be formed from pyruvate as one of the anaplerotic reactions. |
|---|
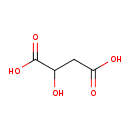
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Hydroxybutanedioic acid | ChEBI | | 2-Hydroxyethane-1,2-dicarboxylic acid | ChEBI | | 2-Hydroxysuccinic acid | ChEBI | | alpha-Hydroxysuccinic acid | ChEBI | | Aepfelsaeure | ChEBI | | Apple acid | ChEBI | | DL-Malic acid | ChEBI | | e296 | ChEBI | | H2Mal | ChEBI | | Hydroxybutanedioic acid | ChEBI | | Hydroxysuccinic acid | ChEBI | | Malate | Kegg | | 2-Hydroxybutanedioate | Generator | | 2-Hydroxyethane-1,2-dicarboxylate | Generator | | 2-Hydroxysuccinate | Generator | | a-Hydroxysuccinate | Generator | | a-Hydroxysuccinic acid | Generator | | alpha-Hydroxysuccinate | Generator | | Α-hydroxysuccinate | Generator | | Α-hydroxysuccinic acid | Generator | | DL-Malate | Generator | | Hydroxybutanedioate | Generator | | Hydroxysuccinate | Generator | | Deoxytetrarate | HMDB | | Deoxytetraric acid | HMDB | | Musashi-NO-ringosan | HMDB | | Pomalus acid | HMDB | | R,S-Malate | HMDB | | R,S-Malic acid | HMDB | | R,SMalate | HMDB | | R,SMalic acid | HMDB | | Malic acid, disodium salt | HMDB | | Malic acid, disodium salt, (R)-isomer | HMDB | | Malic acid, disodium salt, (S)-isomer | HMDB | | Malic acid, monopotassium salt, (+-)-isomer | HMDB | | Malic acid, (R)-isomer | HMDB | | Malic acid, calcium salt, (1:1), (S)-isomer | HMDB | | Malic acid, magnesium salt (2:1) | HMDB | | Calcium (hydroxy-1-malate) hexahydrate | HMDB | | Malic acid, potassium salt, (R)-isomer | HMDB | | Malic acid, sodium salt, (+-)-isomer | HMDB |
|
|---|
| Chemical Formula | C4H6O5 |
|---|
| Average Molecular Weight | 134.0874 |
|---|
| Monoisotopic Molecular Weight | 134.021523302 |
|---|
| IUPAC Name | 2-hydroxybutanedioic acid |
|---|
| Traditional Name | malic acid |
|---|
| CAS Registry Number | 6915-15-7 |
|---|
| SMILES | OC(CC(O)=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C4H6O5/c5-2(4(8)9)1-3(6)7/h2,5H,1H2,(H,6,7)(H,8,9) |
|---|
| InChI Key | BJEPYKJPYRNKOW-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as beta hydroxy acids and derivatives. Beta hydroxy acids and derivatives are compounds containing a carboxylic acid substituted with a hydroxyl group on the C3 carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Hydroxy acids and derivatives |
|---|
| Sub Class | Beta hydroxy acids and derivatives |
|---|
| Direct Parent | Beta hydroxy acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Short-chain hydroxy acid
- Beta-hydroxy acid
- Fatty acid
- Dicarboxylic acid or derivatives
- Alpha-hydroxy acid
- Secondary alcohol
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected and Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (Non-derivatized) | splash10-0002-0920000000-d3afa3ad5c227740eae3 | Spectrum | | GC-MS | GC-MS Spectrum - GC-MS (3 TMS) | splash10-001i-0951000000-1d993823fa816ba3cfb1 | Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0002-0930000000-6a116527910d172eb561 | Spectrum | | GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0002-0920000000-d3afa3ad5c227740eae3 | Spectrum | | GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-001i-0951000000-1d993823fa816ba3cfb1 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9100000000-aba7652c885a434930ef | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-02j9-7191000000-b78c78194b39deee0ca4 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Negative (Annotated) | splash10-03e9-3900000000-6112a756a8c8c7c7cd50 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Negative (Annotated) | splash10-00di-9100000000-b3efe8bce2f89afcff34 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Negative (Annotated) | splash10-00ec-9300000000-c0aaa5301dcac30685db | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kr-7900000000-2a07c36db6acea9015af | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00rj-9200000000-316c7803efd1dfb76523 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00dl-9000000000-a442bcaaacb6f4eec14d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001r-9800000000-03bfaee5de56f72ed927 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0079-9200000000-26a075efe73adc63a189 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-059f-9000000000-a3281a79477ac14e2eae | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0076-9000000000-ad60ea592282d09e4bd8 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 2D NMR | [1H,1H] 2D NMR Spectrum | Not Available | Spectrum | | 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available | Spectrum |
|
|---|