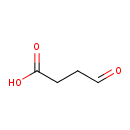
| GC-MS | GC-MS Spectrum - GC-MS (1 MEOX; 1 TMS) | splash10-000i-9400000000-8e1cb554add6ed6c4e35 | Spectrum |
| GC-MS | GC-MS Spectrum - GC-MS (1 MEOX; 1 TMS) | splash10-000i-9300000000-f5403e2e858fded273ac | Spectrum |
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-000i-9400000000-8e1cb554add6ed6c4e35 | Spectrum |
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-000i-9300000000-f5403e2e858fded273ac | Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-000i-9500000000-eb52f4d003b7d5b86b3d | Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-000i-9600000000-463f366ebd0be24a0283 | Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-052f-9000000000-d9618eb947af434dcf2f | Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-05fr-9800000000-40373963cdda851ebfea | Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-000i-9000000000-bf9ebea7e4800559c111 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 1V, negative | splash10-0udi-0900000000-64b3498f7cdd4597da6a | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 1V, negative | splash10-0udi-1900000000-9d47aab2400f1da945bc | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 2V, negative | splash10-0udi-1900000000-94e1ac309574efbe3903 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 2V, negative | splash10-0udi-3900000000-7ec96b67f820176c39d3 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 3V, negative | splash10-0udi-5900000000-7708b3a1c05b69363aa9 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 3V, negative | splash10-0zfr-8900000000-12726b727f621ac971b7 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - n/a 7V, negative | splash10-0089-9000000000-9eb7dd16da8275702c75 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Negative | splash10-0udi-1900000000-567d38fac821ab993d19 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-9200000000-e31b8066f970e1c178d1 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052u-9000000000-76526f6c67d7a67c1d07 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-9000000000-70e85383e59b46bc429f | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-5900000000-f1c2507387f92b6e1e3f | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0zgi-9400000000-ba1b880a90d3ace8beee | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9000000000-971836bb1672ad33106e | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052f-9000000000-06109e02fc0baa708cdc | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052f-9000000000-ce51d68c1c700db8f966 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-d171f37acc222eeea075 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-9100000000-b23feac6075bc2594f5e | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9000000000-aa34fb8bd9988d794431 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9000000000-101491882eb1be877e89 | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available | Spectrum |