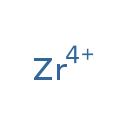Showing metabocard for Zirconium (LMDB00334)
| Record Information | ||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | |||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2016-07-13 19:47:33 UTC | |||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2016-09-23 18:45:12 UTC | |||||||||||||||||||||||||||||||||||||||||||||
| Lmdb | LMDB00334 | |||||||||||||||||||||||||||||||||||||||||||||
| Secondary Accession Numbers | None | |||||||||||||||||||||||||||||||||||||||||||||
| Metabolite Identification | ||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | Zirconium | |||||||||||||||||||||||||||||||||||||||||||||
| Description | The action of Zirconium (Zr) on biological systems presents an enigma. It is ubiquitous, being present in nature in amounts higher than most trace elements. It is taken up by plants from soil and water and accumulated in certain tissues. The entry into animal systems in vivo is related to the mode of exposure and the concentration in the surrounding environment. Retention is initially in soft tissues and then slowly in the bone. The metal is able to cross the blood brain-barrier and is deposited in the brain and the placental barrier to enter milk. Physiologically, it exists as an ion in the body. The daily animal uptake has been known to be as high as 125 mg. The level of toxicity has been found to be moderately low, both in histological and cytological studies. The toxic effects induced by very high concentrations are nonspecific in nature. Despite the presence and retention in relatively high quantities in biological systems, Zr has not yet been associated with any specific metabolic function. Very little information is available about its interaction with the compounds of the genetical systems, such as nucleic acids. Apparently, the metal is neither an essential nor toxic element in the conventional sense. However, the increasing exposure to this element through its increasing use in new materials and following radioactive fallout, has increased the importance of the study of its effects on living organisms. The tetravalent nature of the ionic state and the high stability of the compounds formed are important factors that need to be considered, as also the accumulation of this element in the brain, reminiscent of the relationship between Aluminum and Alzheimer's disease. Zirconium is a metallic element with the atomic number 40 and mol wt 91.99, and belongs to the group IVB and second transition series of the fifth period of Mendeleyev's periodic table. It was named from Arabic zargun, meaning gold color. Discovered in the semiprecious gem zircon, as orthosilicate, by Klaproth in 1789, Zr was isolated as an element by Berzelius in 1824. In its metal form, Zr is hard and resistant to corrosion, heat, and acid. Zirconium behaves like an essential trace metal in the biosphere, although the possibility that it is a natural contaminant with no considerable physiological effects must be considered (PMID: 1283692 , Biol Trace Elem Res. 1992 Dec;35(3):247-71.). | |||||||||||||||||||||||||||||||||||||||||||||
| Structure | ||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| |||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | Zr | |||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight | 91.224 | |||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight | 89.904703679 | |||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | zirconium(4+) ion | |||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | zirconium(4+) ion | |||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 7440-67-7 | |||||||||||||||||||||||||||||||||||||||||||||
| SMILES | [Zr+4] | |||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1S/Zr/q+4 | |||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | GBNDTYKAOXLLID-UHFFFAOYSA-N | |||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of inorganic compounds known as homogeneous transition metal compounds. These are inorganic compounds containing only metal atoms,with the largest atom being a transition metal atom. | |||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Inorganic compounds | |||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Homogeneous metal compounds | |||||||||||||||||||||||||||||||||||||||||||||
| Class | Homogeneous transition metal compounds | |||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Not Available | |||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Homogeneous transition metal compounds | |||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | Not Available | |||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| |||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Not Available | |||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | |||||||||||||||||||||||||||||||||||||||||||||
| Ontology | ||||||||||||||||||||||||||||||||||||||||||||||
| Status | Detected and Quantified | |||||||||||||||||||||||||||||||||||||||||||||
| Origin | Not Available | |||||||||||||||||||||||||||||||||||||||||||||
| Biofunction | Not Available | |||||||||||||||||||||||||||||||||||||||||||||
| Application | Not Available | |||||||||||||||||||||||||||||||||||||||||||||
| Cellular locations | Not Available | |||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||||||||
| State | Not Available | |||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| |||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||||||||
| Spectra |
| |||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations | Not Available | |||||||||||||||||||||||||||||||||||||||||||||
| Biofluid Locations and Tissue Locations |
| |||||||||||||||||||||||||||||||||||||||||||||
| Concentrations | ||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||
| External Links | ||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | HMDB0001475 | |||||||||||||||||||||||||||||||||||||||||||||
| FooDB ID | FDB004189 | |||||||||||||||||||||||||||||||||||||||||||||
| Phenol Explorer ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||
| KNApSAcK ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||
| BiGG ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||
| METLIN ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Zirconium | |||||||||||||||||||||||||||||||||||||||||||||
| Chemspider ID | 103026 | |||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | 33342 | |||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound ID | 115139 | |||||||||||||||||||||||||||||||||||||||||||||
| Kegg Compound ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||
| YMDB ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||
| ECMDB ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | Not Available | |||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | |||||||||||||||||||||||||||||||||||||||||||||
| General References |
| |||||||||||||||||||||||||||||||||||||||||||||