| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-07-13 19:48:14 UTC |
|---|
| Update Date | 2016-09-23 18:45:15 UTC |
|---|
| Lmdb | LMDB00366 |
|---|
| Secondary Accession Numbers | None |
|---|
| Metabolite Identification |
|---|
| Common Name | 17a-Ethynylestradiol |
|---|
| Description | Ethinyl estradiol. A semisynthetic alkylated estradiol with a 17-alpha-ethinyl substitution. It has high estrogenic potency when administered orally, and is often used as the estrogenic component in oral contraceptives. -- Pubchem; estradiol (17-beta estradiol) (also oestradiol) is a sex hormone. Labelled the "female" hormone but also present in males it represents the major estrogen in animals. Critical for sexual functioning, estradiol also supports bone growth. -- Wikipedia; One of the fascinating twists to mammalian sexual differentiation is that estradiol is one of the two active metabolites of testosterone in males (the other being dihydrotestosterone). estradiol cannot be transferred readily from the circulation into the brain. Since fetuses of both sexes are exposed to similarly high levels of maternal estradiol, it can play little role in prenatal sexual differentiation. However, testosterone enters the central nervous system more freely and significant amounts are aromatized to estradiol within the brain of most male mammals, including animals. There is now much evidence that the programming of adult male sexual behavior in "lower mammals," (such as mounting rather than lordosis behavior), is largely dependent on estradiol produced in the central nervous system during prenatal life and early infancy from testosterone. We do not yet know whether this process plays a minimal or significant part in animal sexual behaviors. -- Wikipedia; A synthetic form of estradiol, called ethinyl estradiol is a major component of hormonal contraceptive devices. Combined oral contraceptives contain ethinyl estradiol and a progestin, which both contribute to the inhibition of GnRH, LH, and FSH. The inhibition of these hormones accounts for the ability of combined oral contraceptives or birth control pills to prevent ovulation and thus prevent pregnancy. Other types of hormonal birth control contain only progestins and no ethinyl estradiol. -- Wikipedia. |
|---|
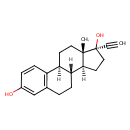
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 17-Ethinyl-3,17-estradiol | ChEBI | | 17-Ethinyl-3,17-oestradiol | ChEBI | | 17-Ethinylestradiol | ChEBI | | 17alpha-Ethinyl estradiol | ChEBI | | Ethinyl estradiol | ChEBI | | Ethinylestradiol | ChEBI | | Ethinyloestradiol | ChEBI | | Ethynyl estradiol | ChEBI | | Estinyl | Kegg | | 17a-Ethinyl estradiol | Generator | | 17Α-ethinyl estradiol | Generator | | 17-Ethynylestradiol | HMDB | | 17-Ethynylestradiol ram | HMDB | | 17-Ethynyloestradiol | HMDB | | 17a-Ethinyl-17b-estradiol | HMDB | | 17a-Ethinylestradiol | HMDB | | 17a-Ethynyl-17b-oestradiol | HMDB | | 17a-Ethynylestradiol-L7b | HMDB | | 17a-Ethynyloestradiol | HMDB | | 17a-Ethynyloestradiol-17b | HMDB | | Alora | HMDB | | Amenoron | HMDB | | Anovlar | HMDB | | Chee-O-gen | HMDB | | Chee-O-genf | HMDB | | Diognat-e | HMDB | | Diogyn e | HMDB | | Diogyn-e | HMDB | | Dyloform | HMDB | | Ertonyl | HMDB | | Esclim | HMDB | | Esteed | HMDB | | Estigyn | HMDB | | Eston-e | HMDB | | Estoral | HMDB | | Estoral {[orion]} | HMDB | | Estorals | HMDB | | Estring | HMDB | | Estrogen | HMDB | | Ethidol | HMDB | | Ethinoral | HMDB | | Ethinylestriol | HMDB | | Ethynylestradiol | HMDB | | Eticyclin | HMDB | | Eticyclol | HMDB | | Eticylol | HMDB | | Etinestrol | HMDB | | Etinestryl | HMDB | | Etinoestryl | HMDB | | Etistradiol | HMDB | | Etivex | HMDB | | Feminone | HMDB | | Fempatch | HMDB | | Follicoral | HMDB | | Ginestrene | HMDB | | Gynodiol | HMDB | | Gynolett | HMDB | | Inestra | HMDB | | Innofem | HMDB | | Kolpolyn | HMDB | | Linoral | HMDB | | Lynoral | HMDB | | Menolyn | HMDB | | Menostar | HMDB | | Neo-estrone | HMDB | | Nogest-S | HMDB | | Novestrol | HMDB | | Oradiol | HMDB | | Orestralyn | HMDB | | Orestrayln | HMDB | | Ovex | HMDB | | Palonyl | HMDB | | Perovex | HMDB | | Primogyn | HMDB | | Primogyn C | HMDB | | Primogyn m | HMDB | | Progynon C | HMDB | | Progynon m | HMDB | | Spanestrin | HMDB | | Thiuram e | HMDB | | Thiuranide | HMDB | | Vagifem | HMDB | | Ylestrol | HMDB | | Ethinyl estradiol, (8 alpha)-isomer | HMDB | | Ethinyl estradiol, (8 alpha,17 alpha)-isomer | HMDB | | Jenapharm, ethinylestradiol | HMDB | | Effik brand OF ethinyl estradiol | HMDB | | Estradiol, ethynyl | HMDB | | Ethinyl estradiol hemihydrate | HMDB | | Ethinyl estradiol, (9 beta,17 alpha)-isomer | HMDB | | Ethinylestradiol jenapharm | HMDB | | Microfollin | HMDB | | Microfollin forte | HMDB | | Estradiol, ethinyl | HMDB | | Ethinyl estradiol, (8 alpha,9 beta,13 alpha,14 beta)-isomer | HMDB | | Ethinyl oestradiol effik | HMDB | | Schering brand OF ethinyl estradiol | HMDB | | Schering-plough brand OF ethinyl estradiol | HMDB | | Ethinyl-oestradiol effik | HMDB | | Hemihydrate, ethinyl estradiol | HMDB | | Jenapharm brand OF ethinyl estradiol | HMDB | | Organon brand OF ethinyl estradiol | HMDB |
|
|---|
| Chemical Formula | C20H24O2 |
|---|
| Average Molecular Weight | 296.4034 |
|---|
| Monoisotopic Molecular Weight | 296.177630012 |
|---|
| IUPAC Name | (1S,10R,11S,14R,15S)-14-ethynyl-15-methyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-2(7),3,5-triene-5,14-diol |
|---|
| Traditional Name | (1S,10R,11S,14R,15S)-14-ethynyl-15-methyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-2(7),3,5-triene-5,14-diol |
|---|
| CAS Registry Number | 57-63-6 |
|---|
| SMILES | [H][C@@]12CC[C@@](O)(C#C)[C@@]1(C)CC[C@]1([H])C3=C(CC[C@@]21[H])C=C(O)C=C3 |
|---|
| InChI Identifier | InChI=1S/C20H24O2/c1-3-20(22)11-9-18-17-6-4-13-12-14(21)5-7-15(13)16(17)8-10-19(18,20)2/h1,5,7,12,16-18,21-22H,4,6,8-11H2,2H3/t16-,17-,18+,19+,20+/m1/s1 |
|---|
| InChI Key | BFPYWIDHMRZLRN-SLHNCBLASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as estrogens and derivatives. These are steroids with a structure containing a 3-hydroxylated estrane. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Estrane steroids |
|---|
| Direct Parent | Estrogens and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Estrogen-skeleton
- 3-hydroxysteroid
- Hydroxysteroid
- 17-hydroxysteroid
- Phenanthrene
- Tetralin
- 1-hydroxy-2-unsubstituted benzenoid
- Benzenoid
- Ynone
- Cyclic alcohol
- Tertiary alcohol
- Acetylide
- Organic oxygen compound
- Organooxygen compound
- Hydrocarbon derivative
- Alcohol
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected and Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-03di-0890000000-5219bc8ac1212313c9c0 | Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-03di-0890000000-5219bc8ac1212313c9c0 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-06ec-0890000000-23c55dc07e698aa701e4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-01t9-3264900000-27be1780fbc6934a75b0 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-000b-0290000000-0ef72fb5e00440b9ff50 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-000i-0960000000-945d82a0ab94f246ac88 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-056s-2910000000-0d38daebb87a39304e91 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI M-80) , Positive | splash10-03di-0890000000-7a166e952207cf7bc1d3 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-0a4i-1790000000-194c2e946cdaab2072ce | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0190000000-075ad04641619da4b2f5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004j-0390000000-70fa13954d5025b402ba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fbc-6980000000-8d3156c6218c2f54df34 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-6490bc65476d939667d6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0090000000-9c8e8277a610eab839c8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00p0-0090000000-87d77005eb0529ab1abb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0090000000-fb34058a34b84424b009 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fba-0890000000-310cbc77999e8d6b02f9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0295-2940000000-a936403339b6f3803c0c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-ebc6f53e480399549577 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0090000000-73e320051b256d09bbb0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014r-0090000000-4c31cf3a8784d6c604f1 | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-03dj-2980000000-831c8190c59f98fc8623 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available | Spectrum |
|
|---|