| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-07-13 19:48:27 UTC |
|---|
| Update Date | 2016-07-19 23:37:05 UTC |
|---|
| Lmdb | LMDB00376 |
|---|
| Secondary Accession Numbers | None |
|---|
| Metabolite Identification |
|---|
| Common Name | Fructosamine |
|---|
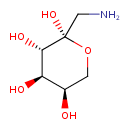
| Description | Fructosamine is a compound which can be considered as the result of a reaction between fructose and ammonia or an amine (with a molecule of water being released). A fructosamine is also formed when carbonyl group of glucose reacts with an amino group of a protein, as the double bond to oxygen moves from the end carbon atom to the next carbon atom and water is released. Fructosamines formed from blood proteins such as serum albumin are known as Glycated Serum Protein (GSP) or Glycated Albumin, and are used to identify the plasma glucose concentration over time and so assess diabetic control. (wikipedia). Glucose molecules are joined to protein molecules to form stable ketoamines, or fructosamines, through glycation, a nonenzymatic mechanism involving a labile Schiff base intermediate and the Amadori rearrangement. The amount of fructosamine in serum is increased in diabetes mellitus owing to the abnormally high concentration of sugar in blood. The concentration of fructosamine in serum thus reflects the degree of glycemic control attained by the diabetic patient and is useful in monitoring the effectiveness of therapy in diabetes over a period of several weeks, in a manner analogous to the determination of glycated hemoglobin. Of the analytical approaches used to measure fructosamine, affinity chromatography with m-aminophenylboronic acid and the nitroblue tetrazolium reduction method appear to be the most practical means for clinical chemists to assay fructosamine quickly, economically, and accurately. Fructosamine values can readily distinguish normal individuals and diabetic patients in good glycemic control from diabetics in poor control. Unlike glycated hemoglobin, which reflects the average blood sugar concentration over the past six to eight weeks, fructosamine reflects the average blood sugar concentration over the past two to three weeks. Thus a clinical advantage is that fructosamine responds more quickly to changes in therapy, thereby allowing for improved glycemic control. Fructosamine is used in conjunction with determinations of blood sugar and (or) of glycated hemoglobin, or by itself, the fructosamine assay can provide clinically useful information for the detection and control of diabetes. (PMID: 3319287 ). |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Amino-1-deoxy-D-fructose | HMDB | | D-Isoglucosamine | HMDB | | D Isoglucosamine | HMDB | | Fructosamine | MeSH |
|
|---|
| Chemical Formula | C6H13NO5 |
|---|
| Average Molecular Weight | 179.1711 |
|---|
| Monoisotopic Molecular Weight | 179.079372531 |
|---|
| IUPAC Name | (2R,3S,4R,5R)-2-(aminomethyl)oxane-2,3,4,5-tetrol |
|---|
| Traditional Name | (2R,3S,4R,5R)-2-(aminomethyl)oxane-2,3,4,5-tetrol |
|---|
| CAS Registry Number | 4429-04-3 |
|---|
| SMILES | NC[C@@]1(O)OC[C@@H](O)[C@@H](O)[C@@H]1O |
|---|
| InChI Identifier | InChI=1S/C6H13NO5/c7-2-6(11)5(10)4(9)3(8)1-12-6/h3-5,8-11H,1-2,7H2/t3-,4-,5+,6-/m1/s1 |
|---|
| InChI Key | IXZISFNWUWKBOM-ARQDHWQXSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as monosaccharides. Monosaccharides are compounds containing one carbohydrate unit not glycosidically linked to another such unit, and no set of two or more glycosidically linked carbohydrate units. Monosaccharides have the general formula CnH2nOn. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Monosaccharides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Monosaccharide
- Oxane
- Hemiacetal
- Secondary alcohol
- Polyol
- Oxacycle
- Organoheterocyclic compound
- Alcohol
- Hydrocarbon derivative
- Organonitrogen compound
- Organopnictogen compound
- Primary aliphatic amine
- Organic nitrogen compound
- Primary amine
- Amine
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Detected and Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001i-9300000000-4706e786831a83f7fdae | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-00di-2920100000-72782c8bd8e44300b317 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01q9-0900000000-fa504cd79cbfd6e45f1f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01q9-2900000000-5e5687353a546e6c16ef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05ar-9200000000-4e65a2b6f1c459eb13c9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00fu-9600000000-5119143995339c78ad83 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00fr-9700000000-41083fc8476785e53998 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-2584bd1445d20f6e5747 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01ot-0900000000-4d89cae6c9a7e59fca4a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03e9-7900000000-cf9880807567a3cb2752 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ox-9000000000-28e351a8717de586ec69 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-6900000000-ffe71ad3e65f7b31f76e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0adi-9200000000-5096012007a6fe6dd9f7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0abc-9000000000-39cc27e8040279e219a0 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|