| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-07-13 19:48:34 UTC |
|---|
| Update Date | 2016-07-19 23:35:52 UTC |
|---|
| Lmdb | LMDB00381 |
|---|
| Secondary Accession Numbers | None |
|---|
| Metabolite Identification |
|---|
| Common Name | Cobalamin |
|---|
| Description | Cobalamin participates in two enzymatic processes in mammalian cells. In the methionine synthase (EC 2.1.1.13) reaction, homocysteine (HCys) is converted to methionine allowing for the "recycling" of 5-methyl-tetrahydrofolate (THF) to N5,10 methylene-THF which is needed for the de novo synthesis of thymidylic acid and ultimately, for DNA formation. Since conversion of N5,10-methylene-THF to N5-methyl-THF is irreversible, cobalamin deficiency "traps" folic acid as N5-methyl-THF. Concurrently, HCys accumulates while methionine decreases, leading to a decrease in S-adenosylmethionine which further limits N5,10-methylene-THF formation by decreasing the synthesis of formyl-THF ("formate starvation"). Decreased methionine and S-adenosylmethionine may limit many methylation reactions including those involving DNA and myelin basic protein. In the methylmalonyl CoA mutase (EC 5.1.99.1) reaction, methylmalonyl CoA, derived from propionic acid synthesized by intestinal bacteria, is converted to succinyl CoA, a precursor for fatty acid and heme synthesis Thus, cobalamin deficiency results in methylmalonic acid (MMA) accumulation. Cobalamin deficiency causes megaloblastic anemia and neurocognitive abnormalities but effects on immune function and bone formation have also been described. Low serum cobalamin levels increase the risk of osteoporosis. Tests for cobalamin deficiency include measurements of 1) total cobalamin; 2) MMA and HCys, as indices of functional cobalamin deficiency; and 3) holotranscobalamin as a measure of the metabolically active fraction of circulating cobalamin. Each approach has significant limitations. Moreover, since the pathogenesis of neurologic dysfunction in cobalamin deficiency remains unclear, these tests may not be reliable markers of neurocognitive impairment. Subtle cobalamin deficiency, defined as elevated metabolite levels usually in asymptomatic patients with low or normal serum cobalamin values, is prevalent in the elderly and has been associated with food cobalamin malabsorption, a disorder characterized by the inability to release vitamin B12 from food or from its binding proteins. Malabsorption is often unrecognized or not investigated. However, because of the potential seriousness of the complications, particularly neuropsychiatric and hematological investigation of all patients who present with vitamin or nutritional deficiency is required. Classic disorders, such as pernicious anemia, are the cause of cobalamin deficiency in only a limited proportion of elderly patients. Epidemiological studies have shown a prevalence of cobalamin deficiency of around 20% in the elderly population of industrialized countries (between 50% and 60%, depending on the definition of cobalamin deficiency used in the study). New routes of cobalamin administration (oral and nasal) are currently being developed, especially the use of oral cobalamin therapy to treat food-cobalamin malabsorption. (PMID: 16814909 , 17822656 ). |
|---|
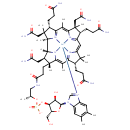
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| alpha-(5,6-Dimethylbenzimidazolyl)cobamide | ChEBI | | Cbl | ChEBI | | Cobalamin (III) | ChEBI | | Cobalamin(1+) | ChEBI | | Cobalamin(III) | ChEBI | | a-(5,6-Dimethylbenzimidazolyl)cobamide | Generator | | Α-(5,6-dimethylbenzimidazolyl)cobamide | Generator | | 5,6-Dimethyl-1-a-D-ribofuranosyl-1H-benzimidazole | HMDB | | 5,6-Dimethyl-1-a-D-ribofuranosylbenzimidazole | HMDB | | 5,6-Dimethyl-1-alpha-delta-ribofuranosyl-1H-benzimidazole | HMDB | | 5,6-Dimethyl-1-alpha-delta-ribofuranosylbenzimidazole | HMDB | | Cob(III)alamin | HMDB | | Cobalamine | HMDB | | Cobinamide ion(1+) dihydrogen phosphate (ester) inner salt 3'-ester | HMDB | | Cobinamide ion(1+) dihydrogen phosphate (ester) inner salt 3'-ester with 5,6-dimethyl-1-alpha-delta-ribofuranosyl-1H-benzimidazole | HMDB | | Hydroxomin | HMDB | | Rubivite | HMDB | | Rubratope-57 | HMDB | | Rubratope-60 | HMDB | | Ruvite | HMDB | | Vitamin b12 | HMDB | | b 12, Vitamin | HMDB | | b12, Vitamin | HMDB | | Cobalamins | HMDB | | Cyanocobalamin | HMDB | | Eritron | HMDB | | Vitamin b 12 | HMDB |
|
|---|
| Chemical Formula | C62H88CoN13O14P |
|---|
| Average Molecular Weight | 1329.3478 |
|---|
| Monoisotopic Molecular Weight | 1328.564331295 |
|---|
| IUPAC Name | (10S,12R,13S,17R,23R,24R,25R,30S,35S,36S,40S,41S,42R,46R)-30,35,40-tris(2-carbamoylethyl)-24,36,41-tris(carbamoylmethyl)-46-hydroxy-12-(hydroxymethyl)-5,6,17,23,28,31,31,36,38,41,42-undecamethyl-15,20-dioxo-11,14,16-trioxa-2lambda5,9,19,26,43lambda5,44lambda5,45lambda5-heptaaza-15lambda5-phospha-1-cobaltadodecacyclo[27.14.1.1^{1,34}.1^{2,9}.1^{10,13}.0^{1,26}.0^{3,8}.0^{23,27}.0^{25,42}.0^{32,44}.0^{39,43}.0^{37,45}]heptatetraconta-2(47),3,5,7,27,29(44),32,34(45),37,39(43)-decaene-2,43,44,45-tetrakis(ylium)-1,1-diuid-15-olate |
|---|
| Traditional Name | (10S,12R,13S,17R,23R,24R,25R,30S,35S,36S,40S,41S,42R,46R)-30,35,40-tris(2-carbamoylethyl)-24,36,41-tris(carbamoylmethyl)-46-hydroxy-12-(hydroxymethyl)-5,6,17,23,28,31,31,36,38,41,42-undecamethyl-15,20-dioxo-11,14,16-trioxa-2lambda5,9,19,26,43lambda5,44lambda5,45lambda5-heptaaza-15lambda5-phospha-1-cobaltadodecacyclo[27.14.1.1^{1,34}.1^{2,9}.1^{10,13}.0^{1,26}.0^{3,8}.0^{23,27}.0^{25,42}.0^{32,44}.0^{39,43}.0^{37,45}]heptatetraconta-2(47),3,5,7,27,29(44),32,34(45),37,39(43)-decaene-2,43,44,45-tetrakis(ylium)-1,1-diuid-15-olate |
|---|
| CAS Registry Number | 13408-78-1 |
|---|
| SMILES | [Co+3].CC(CNC(=O)CCC1(C)C(CC(N)=O)C2[N-]\C1=C(C)/C1=N/C(=C\C3=N\C(=C(C)/C4=NC2(C)C(C)(CC(N)=O)C4CCC(N)=O)\C(C)(CC(N)=O)C3CCC(N)=O)/C(C)(C)C1CCC(N)=O)OP([O-])(=O)OC1C(CO)OC(C1O)N1C=NC2=C1C=C(C)C(C)=C2 |
|---|
| InChI Identifier | InChI=1S/C62H90N13O14P.Co/c1-29-20-39-40(21-30(29)2)75(28-70-39)57-52(84)53(41(27-76)87-57)89-90(85,86)88-31(3)26-69-49(83)18-19-59(8)37(22-46(66)80)56-62(11)61(10,25-48(68)82)36(14-17-45(65)79)51(74-62)33(5)55-60(9,24-47(67)81)34(12-15-43(63)77)38(71-55)23-42-58(6,7)35(13-16-44(64)78)50(72-42)32(4)54(59)73-56;/h20-21,23,28,31,34-37,41,52-53,56-57,76,84H,12-19,22,24-27H2,1-11H3,(H15,63,64,65,66,67,68,69,71,72,73,74,77,78,79,80,81,82,83,85,86);/q;+3/p-2 |
|---|
| InChI Key | NSLAUEAQDBERRV-UHFFFAOYSA-L |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cobalamin derivatives. These are organic compounds containing a corrin ring, a cobalt atom, an a nucleotide moiety. Cobalamin Derivatives are actually derived from vitamin B12. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrapyrroles and derivatives |
|---|
| Sub Class | Corrinoids |
|---|
| Direct Parent | Cobalamin derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cobalamin
- Metallotetrapyrrole skeleton
- 1-ribofuranosylbenzimidazole
- Pentose phosphate
- N-glycosyl compound
- Glycosyl compound
- Monosaccharide phosphate
- Pentose monosaccharide
- Benzimidazole
- Phosphoethanolamine
- Dialkyl phosphate
- Monosaccharide
- N-substituted imidazole
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Benzenoid
- Fatty amide
- Fatty acyl
- Pyrroline
- Pyrrolidine
- Imidazole
- Azole
- Tetrahydrofuran
- Heteroaromatic compound
- Secondary carboxylic acid amide
- Secondary alcohol
- Primary carboxylic acid amide
- Carboxamide group
- Ketimine
- Oxacycle
- Azacycle
- Organic transition metal salt
- Carbene-type 1,3-dipolar compound
- Carboxylic acid derivative
- Alcohol
- Organic nitrogen compound
- Organonitrogen compound
- Organooxygen compound
- Imine
- Primary alcohol
- Organic salt
- Organic cobalt salt
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Carbonyl group
- Organic oxygen compound
- Organic cation
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected and Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-2031000095-b9bf39827ae83761ccb6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4j-2393000063-424c175511ddc5e4d2fe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9740000051-7d6903b2dd8881008344 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0059000000-dec49680276dd7d5a080 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0i00-0092000000-2a13d3fd995ffd79ff7f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-3191000000-20e008a7f11bbe916e61 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|