| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-07-13 19:49:11 UTC |
|---|
| Update Date | 2016-07-20 20:53:31 UTC |
|---|
| Lmdb | LMDB00408 |
|---|
| Secondary Accession Numbers | None |
|---|
| Metabolite Identification |
|---|
| Common Name | Desmosterol |
|---|
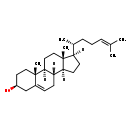
| Description | Desmosterol is an intermediate in the synthesis of cholesterol. Desmosterolosis is a rare autosomal recessive inborn errors of cholesterol synthesis that is caused by defective activity of desmosterol reductase which results in an accumulation of demosterol (DHCR24, EC 1.3.1.72), combines a severe osteosclerotic skeletal dysplasia and includes 2-3 toe syndactyly with Smith-Lemli-Opitz syndrome (SLOS; the biochemical block in SLOS results in decreased cholesterol levels and increased 7-dehydrocholesterol levels). Desmosterolosis is caused by mutation of the 24-dehydrocholesterol reductase gene (DHCR24). Many of the malformations in SLOS and desmosterolosis are consistent with impaired hedgehog function. The hedgehog proteins include Sonic hedgehog (SHH), which plays a major role in midline patterning and limb development. Desmosterolosis, caused by defective activity of desmosterol reductase, combines a severe osteosclerotic skeletal dysplasia. 7-dehydrocholesterol reductase (DHCR7, EC 1.3.1.21) reduces the C7-C8 double bond in the sterol B ring to form cholesterol or desmosterol depending upon the precursor. Desmosterol can be converted to cholesterol by DHCR24. Therefore, SLOS and Desmosterolosis patients invariably have elevated levels of cholesterol precursor's 7-dehydrocholesterol (and its spontaneous isomer 8-dehydrocholesterol) and absent desmosterol. (PMID: 14631207 , 16207203 ). |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 24-Dehydrocholesterol | ChEBI | | 3beta-Cholesta-5,24-dien-3-ol | ChEBI | | Cholesta-5,24-dien-3beta-ol | ChEBI | | 3b-Cholesta-5,24-dien-3-ol | Generator | | 3Β-cholesta-5,24-dien-3-ol | Generator | | Cholesta-5,24-dien-3b-ol | Generator | | Cholesta-5,24-dien-3β-ol | Generator | | Cholest-5,24-dien-3beta-ol | HMDB | | Cholesta-5,24-dien-3-ol | HMDB | | 24 Dehydrocholesterol | HMDB | | Demosterol | HMDB | | (3beta)-Cholesta-5,24-dien-3-ol | HMDB | | (3Β)-cholesta-5,24-dien-3-ol | HMDB | | 24,25-Dehydrocholesterol | HMDB | | Desmosterol | HMDB |
|
|---|
| Chemical Formula | C27H44O |
|---|
| Average Molecular Weight | 384.6377 |
|---|
| Monoisotopic Molecular Weight | 384.33921603 |
|---|
| IUPAC Name | (1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-6-methylhept-5-en-2-yl]tetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-7-en-5-ol |
|---|
| Traditional Name | (1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-6-methylhept-5-en-2-yl]tetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-7-en-5-ol |
|---|
| CAS Registry Number | 313-04-2 |
|---|
| SMILES | [H][C@@]12CC[C@H]([C@H](C)CCC=C(C)C)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@@H](O)CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C27H44O/c1-18(2)7-6-8-19(3)23-11-12-24-22-10-9-20-17-21(28)13-15-26(20,4)25(22)14-16-27(23,24)5/h7,9,19,21-25,28H,6,8,10-17H2,1-5H3/t19-,21+,22+,23-,24+,25+,26+,27-/m1/s1 |
|---|
| InChI Key | AVSXSVCZWQODGV-DPAQBDIFSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cholesterols and derivatives. Cholesterols and derivatives are compounds containing a 3-hydroxylated cholestane core. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Cholestane steroids |
|---|
| Direct Parent | Cholesterols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cholesterol-skeleton
- Cholesterol
- 3-beta-hydroxysteroid
- 3-beta-hydroxy-delta-5-steroid
- Hydroxysteroid
- 3-hydroxysteroid
- 3-hydroxy-delta-5-steroid
- Delta-5-steroid
- Cyclic alcohol
- Secondary alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected and Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-00xr-6973000000-9b8478114c7fec1504f2 | Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-00xr-6973000000-9b8478114c7fec1504f2 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0axr-1109000000-c96b90d5bc5f0ee8db10 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-002f-3105900000-043e100bd5bd92eb1582 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014r-0019000000-9cec9aa1e8ad662bef64 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-2149000000-6218d03e9c19a9f2887f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-02ta-4259000000-713a8956eb12b8b2003d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0009000000-387b878bf983d0b8ac76 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0009000000-2d0cbb4e60d96f291bce | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0gb9-1019000000-37313ec6fa045ceba894 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0009000000-4d7a5f3b63c0fb62f1d7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0009000000-3697eddae534b800a992 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-0009000000-e7c1ee286ab735e93547 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-1009000000-1881c8faacf610d02817 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-7094000000-adf191ce524bfed85be3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-9710000000-dc3e59d9eff8b9729026 | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0avl-7941000000-99b5e69bc97a4056fa8c | Spectrum |
|
|---|