| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-07-13 19:49:24 UTC |
|---|
| Update Date | 2016-09-23 18:45:45 UTC |
|---|
| Lmdb | LMDB00418 |
|---|
| Secondary Accession Numbers | None |
|---|
| Metabolite Identification |
|---|
| Common Name | Raffinose |
|---|
| Description | A trisaccharide occurring in Australian manna (from Eucalyptus spp, Myrtaceae) and in cottonseed meal. Raffinose is a complex carbohydrate, a trisaccharide composed of galactose, fructose, and glucose. It can be found in beans, cabbage, brussels sprouts, broccoli, asparagus, other vegetables, and whole grains. Raffinose is hydrolysed to D-galactose and sucrose by D-galactosidase (D-GAL) (1). D-GAL also hydrolyses other D-galactosides such as stachyose, verbascose, and galactinol [1-O-(D-galactosyl)-myoinositol], if present. The enzyme does not cleave linked galactose, as in lactose. Raffinose is also known as melitose and may be thought of as galactose + sucrose connected via an alpha(1-6) glycosidic linkage and so raffinose can be broken apart into galactose and sucrose via the enzyme alpha-galactosidase. |
|---|
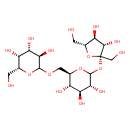
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 6g-alpha-D-Galactosylsucrose | HMDB | | 6g-alpha-delta-Galactosylsucrose | HMDB | | D-(+)-Raffinose | HMDB | | D-Raffinose | HMDB | | delta-(+)-Raffinose | HMDB | | delta-Raffinose | HMDB | | Gossypose | HMDB | | Melitose | HMDB | | Melitriose | HMDB | | Raffinose hydrate | HMDB |
|
|---|
| Chemical Formula | C18H32O16 |
|---|
| Average Molecular Weight | 504.4371 |
|---|
| Monoisotopic Molecular Weight | 504.169034976 |
|---|
| IUPAC Name | (3R,4S,5R,6R)-2-{[(2R,3S,4S,5R)-6-{[(2S,3S,4S,5R)-3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy}-3,4,5-trihydroxyoxan-2-yl]methoxy}-6-(hydroxymethyl)oxane-3,4,5-triol |
|---|
| Traditional Name | (3R,4S,5R,6R)-2-{[(2R,3S,4S,5R)-6-{[(2S,3S,4S,5R)-3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy}-3,4,5-trihydroxyoxan-2-yl]methoxy}-6-(hydroxymethyl)oxane-3,4,5-triol |
|---|
| CAS Registry Number | 512-69-6 |
|---|
| SMILES | OC[C@H]1O[C@@](CO)(OC2O[C@H](COC3O[C@H](CO)[C@H](O)[C@H](O)[C@H]3O)[C@@H](O)[C@H](O)[C@H]2O)[C@@H](O)[C@@H]1O |
|---|
| InChI Identifier | InChI=1S/C18H32O16/c19-1-5-8(22)11(25)13(27)16(31-5)30-3-7-9(23)12(26)14(28)17(32-7)34-18(4-21)15(29)10(24)6(2-20)33-18/h5-17,19-29H,1-4H2/t5-,6-,7-,8+,9-,10-,11+,12+,13-,14-,15+,16?,17?,18+/m1/s1 |
|---|
| InChI Key | MUPFEKGTMRGPLJ-BQCSWRFHSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oligosaccharides. These are carbohydrates made up of 3 to 10 monosaccharide units linked to each other through glycosidic bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Oligosaccharides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Oligosaccharide
- C-glycosyl compound
- Glycosyl compound
- O-glycosyl compound
- Ketal
- Oxane
- Tetrahydrofuran
- Secondary alcohol
- Acetal
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Alcohol
- Primary alcohol
- Hydrocarbon derivative
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0079-5312900000-8c1da6b96f865046dbc2 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0l1i-7432149000-e8883b57e0531dff1cc0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01q9-0901000000-64f2e5bde9015fd5ad69 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03gi-0906000000-fc81b4e448a153802db5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03dl-9800000000-c8990312d1658c9255a1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-2900000000-b81d8d3ee7919e19296a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03fr-0900000000-319ebbeb5b37f8ed7f2d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002e-6900000000-6b955be165071ff0a635 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available | Spectrum |
|
|---|