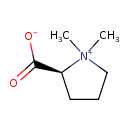| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-07-13 19:50:18 UTC |
|---|
| Update Date | 2016-09-23 18:45:31 UTC |
|---|
| Lmdb | LMDB00458 |
|---|
| Secondary Accession Numbers | None |
|---|
| Metabolite Identification |
|---|
| Common Name | Proline betaine |
|---|
| Description | Proline betaine, also known as stachydrine, belongs to the class of organic compounds known as proline and derivatives. Proline and derivatives are compounds containing proline or a derivative thereof resulting from reaction of proline at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. Proline betaine exists in all living organisms, ranging from bacteria to humans. Proline betaine is found, on average, in the highest concentration within capers (Capparis spinosa). Proline betaine has also been detected, but not quantified in, several different foods, such as soy beans (Glycine max), crosnes (Stachys affinis), domestic pigs (Sus scrofa domestica), limes (Citrus aurantiifolia), and triticales (X Triticosecale rimpaui). This could make proline betaine a potential biomarker for the consumption of these foods. Proline betaine is a secondary metabolite. Secondary metabolites are metabolically or physiologically non-essential metabolites that may serve a role as defense or signalling molecules. In some cases they are simply molecules that arise from the incomplete metabolism of other secondary metabolites. Based on a literature review a significant number of articles have been published on Proline betaine. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (S)-2-Carboxylato-1,1-dimethylpyrrolidinium | ChEBI | | N,N-Dimethyl-L-proline | ChEBI | | Stachydrine | ChEBI | | L-Proline betaine | Kegg | | (2S)-2-Carboxy-1,1-dimethylpyrrolidinium | HMDB | | (S)-2-Carboxy-1,1-dimethylpyrrolidinium | HMDB | | Homostachydrine | HMDB | | Prestwick-08g03 | HMDB | | Dimethylproline | MeSH, HMDB | | Stachydrine chloride | MeSH, HMDB | | Stachydrine chloride, (S)-isomer | MeSH, HMDB | | Stachydrine, (+-)-isomer | MeSH, HMDB | | Proline betaine | ChEBI |
|
|---|
| Chemical Formula | C7H13NO2 |
|---|
| Average Molecular Weight | 143.1836 |
|---|
| Monoisotopic Molecular Weight | 143.094628665 |
|---|
| IUPAC Name | (2S)-1,1-dimethylpyrrolidin-1-ium-2-carboxylate |
|---|
| Traditional Name | stachydrine |
|---|
| CAS Registry Number | 471-87-4 |
|---|
| SMILES | C[N+]1(C)CCC[C@H]1C([O-])=O |
|---|
| InChI Identifier | InChI=1S/C7H13NO2/c1-8(2)5-3-4-6(8)7(9)10/h6H,3-5H2,1-2H3/t6-/m0/s1 |
|---|
| InChI Key | CMUNUTVVOOHQPW-LURJTMIESA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as proline and derivatives. Proline and derivatives are compounds containing proline or a derivative thereof resulting from reaction of proline at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Proline and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Proline or derivatives
- Alpha-amino acid
- L-alpha-amino acid
- Pyrrolidine carboxylic acid
- Pyrrolidine carboxylic acid or derivatives
- N-alkylpyrrolidine
- Tetraalkylammonium salt
- Pyrrolidine
- Quaternary ammonium salt
- Carboxylic acid salt
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Azacycle
- Organoheterocyclic compound
- Organic oxide
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Amine
- Carbonyl group
- Organic salt
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-002e-9000000000-36bbb91342358293711a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 10V, Negative | splash10-0bt9-9200000000-395432f0cf8795dd4cf7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0900000000-ea4782d2282bc28329c0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0007-4900000000-75a3b0df6adc0100b9fe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0aou-9000000000-5df24b6079668b584f79 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0900000000-34921bec4b2fad07f505 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-3900000000-da3a14fcb5a4909b775e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000f-9000000000-7b04a861eee46ac86b66 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-9500000000-135bbe696520934e172e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-9100000000-6d9ce7552b62c3e5d66c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-059w-9100000000-1648e60acdd97fc80a9d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0900000000-bf6e206b443f53ef90b5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0900000000-bf6e206b443f53ef90b5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9200000000-94164ee813c486cb0b96 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|