| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-07-13 19:50:36 UTC |
|---|
| Update Date | 2016-07-20 21:01:25 UTC |
|---|
| Lmdb | LMDB00472 |
|---|
| Secondary Accession Numbers | None |
|---|
| Metabolite Identification |
|---|
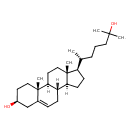
| Common Name | 25-Hydroxycholesterol |
|---|
| Description | 25-Hydroxycholesterol is steroid derivative that suppresses the cleavage of sterol regulatory element binding proteins (SREBPs). It also induces apoptosis through down-regulation of Bcl-2 expression and activation of caspases. 25-Hydroxycholesterol also enhances Interleukin-1 beta (IL-1beta-induced) IL-8 production.(PMID: 17086498 ). 25-hydroxycholesterol is endogenously produced from cholesterol at early time intervals after cholesterol ingestion. It inhibits HMG-CoA reductase and so it also plays a significant role in the in vivo regulation of cholesterol biosynthesis after an acute dietary cholesterol challenge. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (beta)-Cholest-5-ene-3-25-diol | HMDB | | 25-Hydroxy-cholesterol | HMDB | | 25-Hydroxycholest-5-en-3-ol | HMDB | | 5-Cholestene-3beta-25-diol | HMDB | | Cholest-5-en-3beta-25-diol | HMDB | | Cholest-5-ene-3-b,25-diol | HMDB | | Cholest-5-ene-3-beta,25-diol | HMDB | | Cholest-5-ene-3beta-25-diol | HMDB |
|
|---|
| Chemical Formula | C27H46O2 |
|---|
| Average Molecular Weight | 402.6529 |
|---|
| Monoisotopic Molecular Weight | 402.349780716 |
|---|
| IUPAC Name | (2R,5S,10S,14R,15R)-14-[(2R)-6-hydroxy-6-methylheptan-2-yl]-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-7-en-5-ol |
|---|
| Traditional Name | (2R,5S,10S,14R,15R)-14-[(2R)-6-hydroxy-6-methylheptan-2-yl]-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-7-en-5-ol |
|---|
| CAS Registry Number | 2140-46-7 |
|---|
| SMILES | [H]C12CC[C@H]([C@H](C)CCCC(C)(C)O)[C@@]1(C)CCC1([H])[C@@]2([H])CC=C2C[C@@H](O)CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C27H46O2/c1-18(7-6-14-25(2,3)29)22-10-11-23-21-9-8-19-17-20(28)12-15-26(19,4)24(21)13-16-27(22,23)5/h8,18,20-24,28-29H,6-7,9-17H2,1-5H3/t18-,20+,21+,22-,23?,24?,26+,27-/m1/s1 |
|---|
| InChI Key | INBGSXNNRGWLJU-POAVOMIWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cholesterols and derivatives. Cholesterols and derivatives are compounds containing a 3-hydroxylated cholestane core. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Cholestane steroids |
|---|
| Direct Parent | Cholesterols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cholesterol
- Cholesterol-skeleton
- 25-hydroxysteroid
- 3-beta-hydroxy-delta-5-steroid
- Hydroxysteroid
- 3-hydroxy-delta-5-steroid
- 3-hydroxysteroid
- 3-beta-hydroxysteroid
- Delta-5-steroid
- Tertiary alcohol
- Cyclic alcohol
- Secondary alcohol
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxygen compound
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-059l-1009000000-cec357bcbaf7149e29f5 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-001i-2211390000-0527cfa38749e09bd2b6 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kr-0009100000-9e729aebf6add28b22e8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0170-1109000000-3349124bb19a8fd2ee08 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-02di-2239000000-7a23aff71704f1c442ac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0003900000-bc39f53a2f47d9e530b4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ue9-0009600000-14eb751b01166860ee82 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kr-1019000000-981e4ae54c86fe2b7d18 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0000900000-079f76b4d985e4b92c9d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0001900000-25ec6288bc3521fc5e32 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udj-0039300000-b49af36b609e46ef3701 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kr-0309000000-94663290c06fb4528adf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-8669100000-8343de835a906d68edec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-5952000000-e7e8f0bed73a8187daa3 | Spectrum |
|
|---|