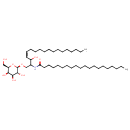| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-07-13 19:52:06 UTC |
|---|
| Update Date | 2016-07-20 21:01:54 UTC |
|---|
| Lmdb | LMDB00539 |
|---|
| Secondary Accession Numbers | None |
|---|
| Metabolite Identification |
|---|
| Common Name | Galactosylceramide (d18:1/20:0) |
|---|
| Description | Galactosylceramides (GalCer) are non-acidic monoglycosphingolipids, i.e. a sphingolipid with one carbohydrate moiety attached to a ceramide unit. They are an intermediate in sphingolipid metabolism and is the second to last step in the synthesis of digalactosylceramidesulfate. GalCer is generated from ceramide via the enzyme UDP-galactose ceramide galactosyltransferase [EC:2.4.1.47]. It can be converted to digalactosylceramide via the enzyme glycosyltransferases [EC 2.4.1.-]. Galactosylceramide is the principal glycosphingolipid in brain tissue, hence the trivial name "cerebroside", which was first conferred on it in 1874. Galactosylceramides are found in all nervous tissues, but they can amount to 2% of the dry weight of grey matter and 12% of white matter. They are major constituents of oligodendrocytes. Synthesis of galactosylceramide takes place on the lumenal surface of the endoplasmic reticulum, although it has free access to the cytosolic surface by an energy-independent flip-flop process. GalCer sits in the extracellular leaflet of cell membranes in nanometer sized domains or rafts. The local clustering of GalCer within rafts is thought to facilitate the initial adhesion of certain viruses, including HIV-1 and bacteria to cells through multivalent interactions between receptor proteins and GalCer. A defect in the degradation of cerbrosides leads to a disorder called Krabbe disease. Krabbe disease (also known as globoid cell leukodystrophy or galactosylceramide lipidosis) is a rare, often fatal degenerative disorder that affects the myelin sheath of the nervous system. Krabbe disease is caused by mutations in the GALC gene, which causes a deficiency of galactosylceramidase. Infants with Krabbe disease are normal at birth. Symptoms begin between the ages of 3 and 6 months with irritability, fevers, limb stiffness, seizures, feeding difficulties, vomiting, and slowing of mental and motor development. There are also juvenile- and adult-onset cases of Krabbe disease, which have similar symptoms but slower progression. In infants, the disease is generally fatal before age 2. Patients with late-onset Krabbe disease tend to have a slower progression of the disease and live significantly longer.Cerebrosides are glycosphingolipids. There are four types of glycosphingolipids, the cerebrosides, sulfatides, globosides and gangliosides. Cerebrosides have a single sugar group linked to ceramide. The most common are galactocerebrosides (containing galactose), the least common are glucocerebrosides (containing glucose). Galactocerebrosides are found predominantly in neuronal cell membranes. In contrast glucocerebrosides are not normally found in membranes. Instead, they are typically intermediates in the synthesis or degradation of more complex glycosphingolipids. Galactocerebrosides are synthesized from ceramide and UDP-galactose. Excess lysosomal accumulation of glucocerebrosides is found in Gaucher disease. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| a-GalCer | HMDB | | alpha-GalCer | HMDB | | Cerebroside | HMDB | | D-Galactosyl-N-acylsphingosine | HMDB | | D-Galactosylceramide | HMDB | | delta-Galactosyl-N-acylsphingosine | HMDB | | delta-Galactosylceramide | HMDB | | Gal-b-cer | HMDB | | Gal-beta-1-1'cer | HMDB | | Gal-beta-cer | HMDB | | Galactocerebroside | HMDB | | Galactosylceramide | HMDB | | GalCer | HMDB | | N-(Eicosanoyl)-1-b-galactosyl-sphing-4-enine | HMDB | | N-(Eicosanoyl)-1-beta-galactosyl-sphing-4-enine | HMDB | | N-[(4Z)-3-Hydroxy-1-{[(2R,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}octadec-4-en-2-yl]icosanimidate | Generator, HMDB |
|
|---|
| Chemical Formula | C44H85NO8 |
|---|
| Average Molecular Weight | 756.1476 |
|---|
| Monoisotopic Molecular Weight | 755.627518701 |
|---|
| IUPAC Name | N-[(4Z)-3-hydroxy-1-{[(2R,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}octadec-4-en-2-yl]icosanamide |
|---|
| Traditional Name | N-[(4Z)-3-hydroxy-1-{[(2R,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}octadec-4-en-2-yl]icosanamide |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CCCCCCCCCCCCCCCCCCCC(=O)NC(CO[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O)C(O)\C=C/CCCCCCCCCCCCC |
|---|
| InChI Identifier | InChI=1S/C44H85NO8/c1-3-5-7-9-11-13-15-17-18-19-20-22-24-26-28-30-32-34-40(48)45-37(36-52-44-43(51)42(50)41(49)39(35-46)53-44)38(47)33-31-29-27-25-23-21-16-14-12-10-8-6-4-2/h31,33,37-39,41-44,46-47,49-51H,3-30,32,34-36H2,1-2H3,(H,45,48)/b33-31-/t37?,38?,39-,41+,42+,43-,44-/m1/s1 |
|---|
| InChI Key | DFELABABMXOKTD-RBULGSFCSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as glycosyl-n-acylsphingosines. Glycosyl-N-acylsphingosines are compounds containing a sphingosine linked to a simple glucosyl moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Sphingolipids |
|---|
| Sub Class | Glycosphingolipids |
|---|
| Direct Parent | Glycosyl-N-acylsphingosines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Glycosyl-n-acylsphingosine
- Fatty acyl glycoside
- Fatty acyl glycoside of mono- or disaccharide
- Alkyl glycoside
- Hexose monosaccharide
- Glycosyl compound
- O-glycosyl compound
- Fatty amide
- Fatty acyl
- Monosaccharide
- N-acyl-amine
- Oxane
- Carboxamide group
- Secondary carboxylic acid amide
- Secondary alcohol
- Acetal
- Carboxylic acid derivative
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Alcohol
- Organic oxygen compound
- Organic nitrogen compound
- Primary alcohol
- Carbonyl group
- Organooxygen compound
- Organonitrogen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_5) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_6) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_4) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_5) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_6) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_7) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_8) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_9) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_10) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_11) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_12) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_13) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_14) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_15) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_4) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-4000051900-a94f51802ae020a6561d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-056r-7200192500-6a86d00ca50b22183721 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000x-9140020000-9b1b099fc3fd3ab558ee | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0000000900-99004b18b82df3527f7c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-5110125900-0c669cc86594822cb215 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0pb9-9257030000-114b940f25b95285ca5c | Spectrum |
|
|---|