| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-07-13 19:52:10 UTC |
|---|
| Update Date | 2016-07-20 20:44:50 UTC |
|---|
| Lmdb | LMDB00542 |
|---|
| Secondary Accession Numbers | None |
|---|
| Metabolite Identification |
|---|
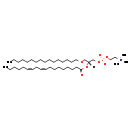
| Common Name | PC(O-16:0/18:2(9Z,12Z)) |
|---|
| Description | PC(O-16:0/18:2(9Z,12Z)) is an ether lipid. Ether lipids are lipids in which one or more of the carbon atoms on glycerol is bonded to an alkyl chain via an ether linkage, as opposed to the usual ester linkage.Phosphatidylcholines are a class of phospholipids which incorporate choline as a headgroup. They are a major component of biological membranes and can be isolated from either egg yolk (in Greek lekithos) or soy beans from which they are mechanically extracted or chemically extracted using hexane.Phosphatidylcholines are such a major component of lecithin, that, in some contexts, the terms are sometime used as synonyms. However, lecithin extract consists of a mixture of phosphatidylcholine and other compounds. It is also used along with sodium taurocholate for simulating fed- and fasted-state biorelevant media in dissolution studies of highly-lipophilic drugs. Phosphatidylcholine is a major constituent of cell membranes, and also plays a role in membrane-mediated cell signalling.Phospholipase D catalyzes the hydrolysis of phosphatidylcholine to form phosphatidic acid (PA), releasing the soluble choline headgroup into the cytosol. Some medical researchers are experimenting with using Phosphatidylcholine in a type of injection that will break down fat cells; to be used as an alternative to liposuction known as Injection lipolysis. (Wikipedia)While most phospholipids have a saturated fatty acid on C-1 and an unsaturated fatty acid on C-2 of the glycerol backbone, the fatty acid distribution at the C-1 and C-2 positions of glycerol within phospholipids is continually in flux, owing to phospholipid degradation and the continuous phospholipid remodeling that occurs while these molecules are in membranes. PCs can be synthesized via three different routes. In one route, choline is activated first by phosphorylation and then by coupling to CDP prior to attachment to phosphatidic acid. PCs can also synthesized by the addition of choline to CDP-activated 1,2-diacylglycerol. A third route to PC synthesis involves the conversion of either PS or PE to PC. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Hexadecyl-2-(9Z,12Z-octadecadienoyl)-sn-glycero-3-phosphocholine | ChEBI | | 2-(9Z,12Z-Octadecadienoyl)-1-hexadecyl-sn-glycero-3-phosphocholine | HMDB | | PC Ae C34:2 | HMDB | | PC(16:0E/18:2(9Z,12Z)) | HMDB | | PC(O-34:2) | HMDB | | [(2R)-3-Hexadecoxy-2-[(9Z,12Z)-octadeca-9,12-dienoyl]oxypropyl] 2-trimethylazaniumylethyl phosphate | HMDB |
|
|---|
| Chemical Formula | C42H82NO7P |
|---|
| Average Molecular Weight | 744.0767 |
|---|
| Monoisotopic Molecular Weight | 743.582890495 |
|---|
| IUPAC Name | (2-{[(2R)-3-(hexadecyloxy)-2-[(9Z,12Z)-octadeca-9,12-dienoyloxy]propyl phosphonato]oxy}ethyl)trimethylazanium |
|---|
| Traditional Name | (2-{[(2R)-3-(hexadecyloxy)-2-[(9Z,12Z)-octadeca-9,12-dienoyloxy]propyl phosphonato]oxy}ethyl)trimethylazanium |
|---|
| CAS Registry Number | 88542-95-4 |
|---|
| SMILES | CCCCCCCCCCCCCCCCOC[C@]([H])(COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCC\C=C/C\C=C/CCCCC |
|---|
| InChI Identifier | InChI=1S/C42H82NO7P/c1-6-8-10-12-14-16-18-20-22-23-25-27-29-31-33-35-42(44)50-41(40-49-51(45,46)48-38-36-43(3,4)5)39-47-37-34-32-30-28-26-24-21-19-17-15-13-11-9-7-2/h14,16,20,22,41H,6-13,15,17-19,21,23-40H2,1-5H3/b16-14-,22-20-/t41-/m1/s1 |
|---|
| InChI Key | IQACMFWAGALEAQ-WESJWMGVSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1-alkyl,2-acylglycero-3-phosphocholines. These are glycerophosphocholines that carry exactly one acyl chain attached to the glycerol moiety through an ester linkage at the O2-position, and one alkyl chain attached through an ether linkage at the O1-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerophospholipids |
|---|
| Sub Class | Glycerophosphocholines |
|---|
| Direct Parent | 1-alkyl,2-acylglycero-3-phosphocholines |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1-alkyl,2-acylglycero-3-phosphocholine
- Phosphocholine
- Fatty acid ester
- Dialkyl phosphate
- Glycerol ether
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Fatty acyl
- Alkyl phosphate
- Tetraalkylammonium salt
- Quaternary ammonium salt
- Carboxylic acid ester
- Carboxylic acid derivative
- Dialkyl ether
- Ether
- Monocarboxylic acid or derivatives
- Organonitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Carbonyl group
- Amine
- Organic nitrogen compound
- Organooxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organic salt
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected and Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01q0-9170321300-dbd7856d52c0aa092c20 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03g0-3290111000-2345c437d6514d4c63c6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0019-7090022000-2630e93789ae7b753477 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-002f-0090003700-619264aad469d6299fc5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004u-1091006100-9abf3093eddcb9a13e76 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004l-4091000000-70d3982de65c2c7f9291 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0000000900-23872d2e94db2d9fdf31 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01ox-0060012900-27c3aa121cb366946f75 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-4091200000-64e10c7f3565c8d5c670 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0000000900-190e542fe7b67b8c2014 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0000000900-190e542fe7b67b8c2014 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00or-0061201900-3adf33f5c1cb71fb8333 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0000000900-414ec66860f431bd7276 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0000000900-414ec66860f431bd7276 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001l-1900341600-1c67b05fa14757dd6ff5 | Spectrum |
|
|---|