| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-07-13 19:52:42 UTC |
|---|
| Update Date | 2016-07-20 21:04:18 UTC |
|---|
| Lmdb | LMDB00567 |
|---|
| Secondary Accession Numbers | None |
|---|
| Metabolite Identification |
|---|
| Common Name | p-Cresol glucuronide |
|---|
| Description | p-Cresol glucuronide is a glucuronide derivative a p-Cresol that is typically excreted in the urine. P-Cresol (the precursor of p-cresol sulfate (PCS) and p-cresol glucuronide (PCG)) is mainly generated as an end product of tyrosine biotransformation by anaerobic intestinal bacteria. During passage through the colonic mucosa and liver, sulfatation and glucuronidation generates p-Cresol sulfate (as the most preponderant metabolite) and p-Cresol glucuronide (at markedly lower concentrations) (PMID: 23826225 ). Cresols are known as methylphenols. Cresols are used to dissolve other chemicals, such as disinfectants and deodorizers. They are also used to make specific chemicals that kill insect pests. Cresol solutions are used as household cleaners and disinfectants such as Lysol. Cresol solutions can also be found in photographic developers. In the past, cresol solutions have been used as antiseptics in surgery, but they have been largely displaced in this role by less toxic compounds. Cresols are found in many foods and in wood and tobacco smoke, crude oil, coal tar, and in brown mixtures such as creosote, cresolene and cresylic acids, which are wood preservatives. Microbes in the soil and water produce cresols when they break down materials in the environment. Most exposures to cresols are at very low levels that are not harmful. When cresols are breathed, ingested, or applied to the skin at very high levels, they can be very harmful. Effects observed in people include irritation and burning of skin, eyes, mouth, and throat; abdominal pain and vomiting. Cresols are also a chemical component found in Sharpie Markers. P-cresol is a major component in pig odor. |
|---|
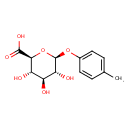
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2S,3S,4S,5R,6S)-3,4,5-Trihydroxy-6-(4-methylphenoxy)oxane-2-carboxylic acid | ChEBI | | Cresol glucuronide | ChEBI | | Cresyl glucuronide | ChEBI | | Cresylglucuronide | ChEBI | | p-Cresyl glucuronide | ChEBI | | p-Cresyl-beta-D-glucuronide | ChEBI | | p-Cresylglucuronide | ChEBI | | (2S,3S,4S,5R,6S)-3,4,5-Trihydroxy-6-(4-methylphenoxy)oxane-2-carboxylate | Generator | | p-Cresyl-b-D-glucuronide | Generator | | p-Cresyl-β-D-glucuronide | Generator | | 4-Methylphenyl beta-D-glucopyranosiduronate | HMDB | | 4-Methylphenyl beta-D-glucopyranosiduronic acid | HMDB | | pCG | HMDB | | 4-Cresylglucuronide | HMDB | | p-Tolyl b-D-glucuronide | HMDB | | p-Tolyl β-D-glucuronide | HMDB | | p-Cresol glucuronide | ChEBI |
|
|---|
| Chemical Formula | C13H16O7 |
|---|
| Average Molecular Weight | 284.2619 |
|---|
| Monoisotopic Molecular Weight | 284.089602866 |
|---|
| IUPAC Name | (2S,3S,4S,5R,6S)-3,4,5-trihydroxy-6-(4-methylphenoxy)oxane-2-carboxylic acid |
|---|
| Traditional Name | (2S,3S,4S,5R,6S)-3,4,5-trihydroxy-6-(4-methylphenoxy)oxane-2-carboxylic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CC1=CC=C(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C13H16O7/c1-6-2-4-7(5-3-6)19-13-10(16)8(14)9(15)11(20-13)12(17)18/h2-5,8-11,13-16H,1H3,(H,17,18)/t8-,9-,10+,11-,13+/m0/s1 |
|---|
| InChI Key | JPAUCQAJHLSMQW-XPORZQOISA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenolic glycosides. These are organic compounds containing a phenolic structure attached to a glycosyl moiety. Some examples of phenolic structures include lignans, and flavonoids. Among the sugar units found in natural glycosides are D-glucose, L-Fructose, and L rhamnose. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Phenolic glycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenolic glycoside
- 1-o-glucuronide
- O-glucuronide
- Glucuronic acid or derivatives
- O-glycosyl compound
- Phenoxy compound
- Phenol ether
- Beta-hydroxy acid
- Toluene
- Monocyclic benzene moiety
- Hydroxy acid
- Monosaccharide
- Benzenoid
- Oxane
- Pyran
- Secondary alcohol
- Oxacycle
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organoheterocyclic compound
- Acetal
- Polyol
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-9740000000-72c74a4f612ab1d96b3c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-0a4i-3111590000-c4085ee51e3627835f9c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-8900000000-125953bb33ef62310b55 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9500000000-c65eb13127918057773b | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 10V, Negative | splash10-03e9-4920000000-31cc7062997a091bd192 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4r-0980000000-0c28ed49edf3ac01b1e4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0910000000-9e86725c906b8cb1ca73 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a6r-9700000000-7c668dc4ccf5c2f1df52 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-053r-1790000000-8e41a0062d8b43c11986 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-1920000000-2c0bc993f06c27f10d87 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-5900000000-078071ef7d6ab4f0ed4c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05n0-0690000000-21c7e95f0396cfbeb2cc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-1920000000-d2d499346dfdb60bcd5c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9500000000-5890de451e5c75c51696 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-053r-0790000000-26241f9e17537357950e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-4910000000-9f376fa7de7498124a4b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9600000000-469910059aa1c2c999fd | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| General References | - Liabeuf S, Glorieux G, Lenglet A, Diouf M, Schepers E, Desjardins L, Choukroun G, Vanholder R, Massy ZA: Does p-cresylglucuronide have the same impact on mortality as other protein-bound uremic toxins? PLoS One. 2013 Jun 24;8(6):e67168. doi: 10.1371/journal.pone.0067168. Print 2013. [23826225 ]
|
|---|