| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-07-13 19:54:37 UTC |
|---|
| Update Date | 2016-07-20 21:02:24 UTC |
|---|
| Lmdb | LMDB00649 |
|---|
| Secondary Accession Numbers | None |
|---|
| Metabolite Identification |
|---|
| Common Name | Aminophylline |
|---|
| Description | Aminophylline belongs to the class of organic compounds known as monoalkylamines. These are organic compounds containing an primary aliphatic amine group. The majority of aminophylline medications are discontinued and the remaining medications on the market are in short supply. Similar to other theophyllines, aminophylline is indicated for the treatment of lung diseases such as asthma, chronic bronchitis, and COPD. Aminophylline is a drug which is used for the treatment of bronchospasm due to asthma, emphysema and chronic bronchitis. Theophylline also binds to the adenosine A2B receptor and blocks adenosine mediated bronchoconstriction. Aminophylline is an extremely weak basic (essentially neutral) compound (based on its pKa). In humans, aminophylline is involved in caffeine metabolism. It relaxes certain smooth muscles in the bronchi, produces diuresis, and causes an increase in gastric secretion. After ingestion, theophylline is released from aminophylline, and theophylline relaxes the smooth muscle of the bronchial airways and pulmonary blood vessels and reduces airway responsiveness to histamine, methacholine, adenosine, and allergen. In inflammatory states, theophylline activates histone deacetylase to prevent transcription of inflammatory genes that require the acetylation of histones for transcription to begin. Aminophylline is a drug combination that contains theophylline and ethylenediamine in a 2:1 ratio. Theophylline competitively inhibits type III and type IV phosphodiesterase (PDE), the enzyme responsible for breaking down cyclic AMP in smooth muscle cells, possibly resulting in bronchodilation. |
|---|
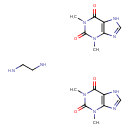
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Somophyllin | Kegg | | Theophylline ethylenediamine | Kegg | | Aminophyllin | HMDB, MeSH | | Aminophylline anhydrous | HMDB | | Aminophylline dihydrate | HMDB | | Aminophylline dye free | HMDB | | Carine | MeSH | | Diaphyllin | MeSH | | Drafilyn | MeSH | | Duraphyllin | MeSH | | Euphyllin retard | MeSH | | Mini-lix | MeSH | | Mundiphyllin retard | MeSH | | Tari-dog | MeSH | | Theophyllamin jenapharm | MeSH | | Aminodur | MeSH | | Clonofilin | MeSH | | Corophyllin | MeSH | | Euphylline | MeSH | | Novophyllin | MeSH | | Phyllocontin | MeSH | | Truphylline | MeSH | | Afonilum | MeSH | | Cardophyllin | MeSH | | Euphyllin | MeSH | | Godafilin | MeSH | | Theophyllamine | MeSH | | Theophyllin edaratiopharm | MeSH | | Aminophylline DF | MeSH | | Ethylenediamine, theophylline | MeSH | | Eufilina | MeSH | | Eufilina venosa | MeSH | | Mundiphyllin | MeSH | | Phyllotemp | MeSH | | Theophyllin eda ratiopharm | MeSH | | Theophyllin eda-ratiopharm | MeSH |
|
|---|
| Chemical Formula | C16H24N10O4 |
|---|
| Average Molecular Weight | 420.4264 |
|---|
| Monoisotopic Molecular Weight | 420.198199306 |
|---|
| IUPAC Name | bis(1,3-dimethyl-2,3,6,7-tetrahydro-1H-purine-2,6-dione); ethane-1,2-diamine |
|---|
| Traditional Name | ethylenediamine; bis(theophylline) |
|---|
| CAS Registry Number | 317-34-0 |
|---|
| SMILES | NCCN.CN1C2=C(NC=N2)C(=O)N(C)C1=O.CN1C2=C(NC=N2)C(=O)N(C)C1=O |
|---|
| InChI Identifier | InChI=1S/2C7H8N4O2.C2H8N2/c2*1-10-5-4(8-3-9-5)6(12)11(2)7(10)13;3-1-2-4/h2*3H,1-2H3,(H,8,9);1-4H2 |
|---|
| InChI Key | FQPFAHBPWDRTLU-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as monoalkylamines. These are organic compounds containing an primary aliphatic amine group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic nitrogen compounds |
|---|
| Class | Organonitrogen compounds |
|---|
| Sub Class | Amines |
|---|
| Direct Parent | Monoalkylamines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Organopnictogen compound
- Hydrocarbon derivative
- Primary aliphatic amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-001i-1900000000-f6d60952fa154f363515 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-001i-1900000000-f6d60952fa154f363515 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0000900000-d88bcbeef6b136a4a7cd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0000900000-d88bcbeef6b136a4a7cd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-0000900000-d88bcbeef6b136a4a7cd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0000900000-7469b5fd344d49cea9ac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0000900000-7469b5fd344d49cea9ac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-0000900000-7469b5fd344d49cea9ac | Spectrum |
|
|---|