| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-07-13 19:55:25 UTC |
|---|
| Update Date | 2016-07-20 21:02:43 UTC |
|---|
| Lmdb | LMDB00685 |
|---|
| Secondary Accession Numbers | None |
|---|
| Metabolite Identification |
|---|
| Common Name | Succinic anhydride |
|---|
| Description | Succinic anhydride, also called dihydro-2,5-furandione, is an organic compound with the molecular formula C4H4O3. It is the acid anhydride of succinic acid. Succinic anhydride has been shown to exhibit antibiotic function (PMID 4840443 ). Succinic anhydride belongs to the family of Oxolanes. These are organic compounds containing an oxolane (tetrahydrofuran) ring, which is a saturated aliphatic five-member ring containing one oxygen and five carbon atoms. |
|---|
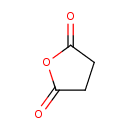
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,5-Diketotetrahydrofuran | ChEBI | | 2,5-Dioxotetrahydrofuran | ChEBI | | Bernsteinsaeureanhydrid | ChEBI | | Butanedioic anhydride | ChEBI | | Dihydro-2,5-furandione | ChEBI | | Dihydrofuran-2,5-dione | ChEBI | | Succinic acid anhydride | ChEBI | | Succinyl anhydride | ChEBI | | Succinyl oxide | ChEBI | | Tetrahydro-2,5-dioxofuran | ChEBI | | Tetrahydro-2,5-furandione | ChEBI | | Succinate anhydride | Generator | | 2,5(3H,4H)-Furandione | HMDB | | 2,5-Furandione, dihydro-, mono-C11-13-alkenyl derivs. | HMDB | | 2-Alkenyl (C11-C13) succinic acid anhydride | HMDB | | Butanedioic acid,anhydride succinic anhydride | HMDB | | Dihydro-2, 5-furandione | HMDB | | Dihydro-2,5-diketotetrahydrofuran | HMDB | | Dihydro-furan-2,5-dione | HMDB | | Oxolane-2,5-dione | HMDB | | Rikacid sa | HMDB | | SAA | HMDB | | Succinic anhydride treated bovine serum albumin | HMDB | | Succinic anhydride treated bsa | HMDB | | Succinyl peroxide | HMDB | | Succinyloxide | HMDB | | Tetrahydro-2, 5-dioxofuran | HMDB |
|
|---|
| Chemical Formula | C4H4O3 |
|---|
| Average Molecular Weight | 100.0728 |
|---|
| Monoisotopic Molecular Weight | 100.016043994 |
|---|
| IUPAC Name | oxolane-2,5-dione |
|---|
| Traditional Name | succinic anhydride |
|---|
| CAS Registry Number | 108-30-5 |
|---|
| SMILES | O=C1CCC(=O)O1 |
|---|
| InChI Identifier | InChI=1S/C4H4O3/c5-3-1-2-4(6)7-3/h1-2H2 |
|---|
| InChI Key | RINCXYDBBGOEEQ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dicarboxylic acids and derivatives. These are organic compounds containing exactly two carboxylic acid groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Dicarboxylic acids and derivatives |
|---|
| Direct Parent | Dicarboxylic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dicarboxylic acid or derivatives
- Tetrahydrofuran
- Carboxylic acid anhydride
- Lactone
- Oxacycle
- Organoheterocyclic compound
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0kdi-9200000000-7aa5270b2ac5ed677ac4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-2900000000-b6c9d054dced9b9c7d79 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-4900000000-f15e0d8dde55d48b1642 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9000000000-751ce6ded2976303a3ea | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-9000000000-fce94dd02c8af5927d04 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052b-9000000000-64e15626f36dab29d672 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0pb9-9000000000-d647422e00ac0f19d689 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-9000000000-bd4c8170b9ee3c19f59a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052b-9000000000-c55763477e5bb8a6b707 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0pb9-9000000000-a693fcab8c2aa97ff11c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0zfr-9700000000-4d81f50d0ea48e8c13dd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4l-9100000000-23d4f9891f64c49c5789 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-9000000000-58e61e6d42bb2d9c8fe8 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|