| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-07-13 19:55:35 UTC |
|---|
| Update Date | 2016-09-23 18:45:45 UTC |
|---|
| Lmdb | LMDB00693 |
|---|
| Secondary Accession Numbers | None |
|---|
| Metabolite Identification |
|---|
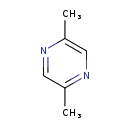
| Common Name | 2,5-Dimethylpyrazine |
|---|
| Description | 2,5-Dimethylpyrazine belongs to the class of organic compounds known as pyrazines. Pyrazines are compounds containing a pyrazine ring, which is a six-member aromatic heterocycle, that consists of two nitrogen atoms (at positions 1 and 4) and four carbon atoms. 2,5-Dimethylpyrazine exists in all eukaryotes, ranging from yeast to plants to humans. 2,5-Dimethylpyrazine is a cocoa, grass, and medicinal tasting compound. 2,5-Dimethylpyrazine is found, on average, in the highest concentration within kohlrabis (Brassica oleracea var. gongylodes). 2,5-Dimethylpyrazine has also been detected, but not quantified in, several different foods, such as breakfast cereal, mollusks, black tea, crustaceans, and herbal tea. This could make 2,5-dimethylpyrazine a potential biomarker for the consumption of these foods. Based on a literature review very few articles have been published on 2,5-Dimethylpyrazine. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 25-Dimethyl-pyrazine | ChEMBL, HMDB | | 2, 5-Dimethylpyrazine | HMDB | | 2,5-Dimethyl pyrazine | HMDB | | 2,5-Dimethyl-1,4-diazine | HMDB | | 2,5-Dimethyl-pyrazine | HMDB | | 2,5-Dimethylparadiazine | HMDB | | 2,5-Dimethylpiazine | HMDB | | FEMA 3272 | HMDB | | PYRAZINE,2,5-dimethyl | HMDB | | 2,6-Dimethylpyrazine | MeSH | | 2,5-DMP | MeSH |
|
|---|
| Chemical Formula | C6H8N2 |
|---|
| Average Molecular Weight | 108.1411 |
|---|
| Monoisotopic Molecular Weight | 108.068748266 |
|---|
| IUPAC Name | 2,5-dimethylpyrazine |
|---|
| Traditional Name | 2,5-dimethylpyrazine |
|---|
| CAS Registry Number | 123-32-0 |
|---|
| SMILES | CC1=CN=C(C)C=N1 |
|---|
| InChI Identifier | InChI=1S/C6H8N2/c1-5-3-8-6(2)4-7-5/h3-4H,1-2H3 |
|---|
| InChI Key | LCZUOKDVTBMCMX-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyrazines. Pyrazines are compounds containing a pyrazine ring, which is a six-member aromatic heterocycle, that consists of two nitrogen atoms (at positions 1 and 4) and four carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Diazines |
|---|
| Sub Class | Pyrazines |
|---|
| Direct Parent | Pyrazines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyrazine
- Heteroaromatic compound
- Azacycle
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-052f-9300000000-081f35e3a3380ed2698b | Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-052f-9300000000-081f35e3a3380ed2698b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-8900000000-2ada7e902f3d0e2b31e9 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0a4i-4900000000-7e680d62ffa714f80403 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0900000000-9b8f23be4c9d8c94f24d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-1900000000-b0964dc373b182b8f4c4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0k9x-9000000000-dc3ffe8529561fb7b1cb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0900000000-032c8099a4abe667a4b9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0900000000-58fa248974f5ade4ee46 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-7900000000-890f0c8c1e9a4f4bf6ff | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0900000000-1d4d8b4513a018558199 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-5900000000-53b7bc7c89378de54482 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-d47061f2d3232fef8318 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-3900000000-a2cd546c692d476da756 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a59-9400000000-696590183181b11c1a14 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udl-9000000000-c5a37365c74d0ec83537 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|