| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-07-30 18:17:56 UTC |
|---|
| Update Date | 2016-08-01 19:17:23 UTC |
|---|
| Lmdb | LMDB00776 |
|---|
| Secondary Accession Numbers | None |
|---|
| Metabolite Identification |
|---|
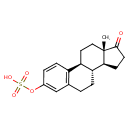
| Common Name | Estrone sulfate |
|---|
| Description | Estrone sulfate, also known as conestoral or premarin, belongs to the class of organic compounds known as sulfated steroids. These are sterol lipids containing a sulfate group attached to the steroid skeleton. Estrone sulfate is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-Hydroxyestra-1,3,5(10)-trien-17-one hydrogen sulphate | ChEBI | | Estrone hydrogen sulfate | ChEBI | | Estrone sulphate | ChEBI | | 3-Hydroxyestra-1,3,5(10)-trien-17-one hydrogen sulfate | Generator | | 3-Hydroxyestra-1,3,5(10)-trien-17-one hydrogen sulfuric acid | Generator | | 3-Hydroxyestra-1,3,5(10)-trien-17-one hydrogen sulphuric acid | Generator | | Estrone hydrogen sulfuric acid | Generator | | Estrone hydrogen sulphate | Generator | | Estrone hydrogen sulphuric acid | Generator | | Estrone sulfuric acid | Generator | | Estrone sulphuric acid | Generator | | Conestoral | HMDB | | Estrogenic substances | HMDB | | Estrone | HMDB | | Estrone hydrogen sulfate | HMDB | | Estrone hydrogen sulphate | HMDB | | Estrone 3-sulfate | HMDB | | Estrone 3-sulphate | HMDB | | Estrone sulfate sodium | HMDB | | Estrone sulphate sodium | HMDB | | Estrone-3-sulfate | HMDB | | Estrone-3-sulphate | HMDB | | Premarin | HMDB | | Sodium estrone 3-monosulfate | HMDB | | Sodium estrone 3-monosulphate | HMDB | | Sodium estrone 3-sulfate | HMDB | | Sodium estrone 3-sulphate | HMDB | | Estrone sulfate, sodium salt | HMDB | | Potassium estrone sulfate | HMDB | | Sodium estrone sulfate | HMDB | | Estrone sulfate, ammonium salt | HMDB | | Oestrone sulphate | HMDB | | Evex | HMDB | | Estrone sulfate, 16-(14)C-labeled | HMDB | | Estrone sulfate, 14C-labeled | HMDB | | Estrone sulfate, potassium salt | HMDB |
|
|---|
| Chemical Formula | C18H22O5S |
|---|
| Average Molecular Weight | 350.429 |
|---|
| Monoisotopic Molecular Weight | 350.118794504 |
|---|
| IUPAC Name | [(1S,10R,11S,15S)-15-methyl-14-oxotetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-2(7),3,5-trien-5-yl]oxidanesulfonic acid |
|---|
| Traditional Name | Ogen |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | C[C@]12CC[C@H]3[C@@H](CCC4=C3C=CC(OS(O)(=O)=O)=C4)[C@@H]1CCC2=O |
|---|
| InChI Identifier | InChI=1S/C18H22O5S/c1-18-9-8-14-13-5-3-12(23-24(20,21)22)10-11(13)2-4-15(14)16(18)6-7-17(18)19/h3,5,10,14-16H,2,4,6-9H2,1H3,(H,20,21,22)/t14-,15-,16+,18+/m1/s1 |
|---|
| InChI Key | JKKFKPJIXZFSSB-CBZIJGRNSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sulfated steroids. These are sterol lipids containing a sulfate group attached to the steroid skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Sulfated steroids |
|---|
| Direct Parent | Sulfated steroids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Sulfated steroid skeleton
- Estrane-skeleton
- 17-oxosteroid
- Oxosteroid
- Phenanthrene
- Arylsulfate
- Tetralin
- Benzenoid
- Sulfuric acid ester
- Sulfuric acid monoester
- Sulfate-ester
- Organic sulfuric acid or derivatives
- Ketone
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Carbonyl group
- Organic oxygen compound
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0079-1195000000-45f680d267db2b386dfb | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0019000000-66837890a45e12ff4df5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ul0-1298000000-be851ade10d052513145 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0zi0-5934000000-d4c5c30541fd04c44e38 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0019000000-45855a16f86d3580ba3c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0093000000-18cc1497d0c7397e16af | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0gc0-5090000000-83bfa06ed14805d78102 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-e8b485c62cd4477fbfc9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-9003000000-98936cb1b9953748d5a9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-7192000000-e9e53d190e852f9b5b1f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0019000000-d7902d7f420476e65a84 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ue9-2189000000-15572bc45f5db3a69da1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gx1-6693000000-29c27e485a34d776e412 | Spectrum |
|
|---|