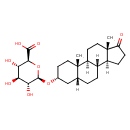| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-07-30 18:17:57 UTC |
|---|
| Update Date | 2016-08-01 19:17:23 UTC |
|---|
| Lmdb | LMDB00777 |
|---|
| Secondary Accession Numbers | None |
|---|
| Metabolite Identification |
|---|
| Common Name | Etiocholanolone glucuronide |
|---|
| Description | Etiocholanolone glucuronide, also known as androsterone glucosiduronate, belongs to the class of organic compounds known as steroid glucuronide conjugates. These are sterol lipids containing a glucuronide moiety linked to the steroid skeleton. Thus, etiocholanolone glucuronide is considered to be a steroid conjugate lipid molecule. Etiocholanolone glucuronide is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3alpha-Hydroxyetiocholan-17-one 3-glucosiduronic acid | ChEBI | | 3alpha-Hydroxyetiocholan-17-one 3-glucuronide | ChEBI | | Etiocholan-3alpha-ol-17-one 3-glucuronide | ChEBI | | Etiocholan-3alpha-ol-17-one 3-glucuronoside | ChEBI | | 3a-Hydroxyetiocholan-17-one 3-glucosiduronate | Generator | | 3a-Hydroxyetiocholan-17-one 3-glucosiduronic acid | Generator | | 3alpha-Hydroxyetiocholan-17-one 3-glucosiduronate | Generator | | 3Α-hydroxyetiocholan-17-one 3-glucosiduronate | Generator | | 3Α-hydroxyetiocholan-17-one 3-glucosiduronic acid | Generator | | 3a-Hydroxyetiocholan-17-one 3-glucuronide | Generator | | 3Α-hydroxyetiocholan-17-one 3-glucuronide | Generator | | Etiocholan-3a-ol-17-one 3-glucuronide | Generator | | Etiocholan-3α-ol-17-one 3-glucuronide | Generator | | Etiocholan-3a-ol-17-one 3-glucuronoside | Generator | | Etiocholan-3α-ol-17-one 3-glucuronoside | Generator | | 17-Oxoandrostan-3-yl hexopyranosiduronic acid | HMDB | | 3alpha-Hydroxy-5beta-androstan-17-one 3-glucuronide | HMDB | | 5Alpha.-androstan-3.alpha.-ol-17-one glucoronide | HMDB | | 5beta-Androstan-3alpha-ol-17-one 3-glucuronide | HMDB | | Etiocholanolone 3-glucuronide | HMDB | | Androsterone glucosiduronate | HMDB | | Androsterone glucuronide | HMDB | | Androsterone glucuronide, (3beta,5alpha)-isomer | HMDB | | Androsterone glucuronide, (beta-D)-isomer | HMDB | | Androsterone glucuronide, sodium salt, (3alpha,5alpha)-isomer | HMDB | | Androsterone glucuronide, sodium salt, (3alpha,5beta)-isomer | HMDB | | (3alpha,5alpha)-17-Oxoandrostan-3-yl beta-D-glucopyranosiduronic acid | HMDB |
|
|---|
| Chemical Formula | C25H38O8 |
|---|
| Average Molecular Weight | 466.5644 |
|---|
| Monoisotopic Molecular Weight | 466.256668192 |
|---|
| IUPAC Name | (2S,3S,4S,5R,6R)-6-{[(1S,2S,5R,7R,10R,11S,15S)-2,15-dimethyl-14-oxotetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-5-yl]oxy}-3,4,5-trihydroxyoxane-2-carboxylic acid |
|---|
| Traditional Name | androsterone glucuronide |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@]2([H])C[C@@H](CC[C@]12C)O[C@@H]1O[C@@H]([C@@H](O)[C@H](O)[C@H]1O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C25H38O8/c1-24-9-7-13(32-23-20(29)18(27)19(28)21(33-23)22(30)31)11-12(24)3-4-14-15-5-6-17(26)25(15,2)10-8-16(14)24/h12-16,18-21,23,27-29H,3-11H2,1-2H3,(H,30,31)/t12-,13-,14+,15+,16+,18+,19+,20-,21+,23-,24+,25+/m1/s1 |
|---|
| InChI Key | VFUIRAVTUVCQTF-SDHZCXLISA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as steroid glucuronide conjugates. These are sterol lipids containing a glucuronide moiety linked to the steroid skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Steroidal glycosides |
|---|
| Direct Parent | Steroid glucuronide conjugates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Steroid-glucuronide-skeleton
- Androstane-skeleton
- Oxosteroid
- 17-oxosteroid
- 1-o-glucuronide
- O-glucuronide
- Glucuronic acid or derivatives
- Hexose monosaccharide
- Glycosyl compound
- O-glycosyl compound
- Beta-hydroxy acid
- Monosaccharide
- Pyran
- Oxane
- Hydroxy acid
- Ketone
- Secondary alcohol
- Acetal
- Carboxylic acid derivative
- Carboxylic acid
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Monocarboxylic acid or derivatives
- Hydrocarbon derivative
- Organic oxygen compound
- Organic oxide
- Carbonyl group
- Organooxygen compound
- Alcohol
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-007a-6254900000-3224ce201f4200b35e4c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00r7-0190600000-3fdda78a1e92f8b5b7b2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-006x-0290000000-51d6fe94f1974c92c97f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00ec-0590000000-71f03526102b61380a88 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01bi-1260900000-703dccf114629fa639b7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-1290200000-1db7014540df9034a06e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-4190000000-b2d4d7c7847df524456f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0000900000-a63f64862ace09c4006b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014r-5241900000-384ea6662fc4abc18a4b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9032100000-351b0f7123639e6a7443 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0000900000-80c326e64042176a4be8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05fs-0792300000-a93b9d1cdd42d4c7cde2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05bb-1904000000-0daa4e3ba6eed62c511b | Spectrum |
|
|---|