| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-07-30 18:57:15 UTC |
|---|
| Update Date | 2016-08-01 19:17:41 UTC |
|---|
| Lmdb | LMDB00822 |
|---|
| Secondary Accession Numbers | None |
|---|
| Metabolite Identification |
|---|
| Common Name | 10-Deacetyl-2-debenzoylbaccatin III |
|---|
| Description | 10-deacetyl-2-debenzoylbaccatin III belongs to the class of organic compounds known as taxanes and derivatives. These are diterpenoids with a structure based either on the taxane skeleton, or a derivative thereof. In term of phytochemistry, several derivatives of the taxane skeleton exist: 2(3->20)-abeotaxane, 3,11-cyclotaxane, 11(15->1),11(10->9)-abeotaxane, 3,8-seco-taxane, and 11(15->1)-abeotaxane, among others. More complex skeletons have been found recently, which include the taxane-derived [3.3.3] propellane ring system. 10-deacetyl-2-debenzoylbaccatin III is an extremely weak basic (essentially neutral) compound (based on its pKa). |
|---|
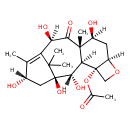
| Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C22H32O9 |
|---|
| Average Molecular Weight | 440.489 |
|---|
| Monoisotopic Molecular Weight | 440.20463261 |
|---|
| IUPAC Name | (1S,2S,3R,4S,7R,9S,10S,12R,15S)-1,2,9,12,15-pentahydroxy-10,14,17,17-tetramethyl-11-oxo-6-oxatetracyclo[11.3.1.0^{3,10}.0^{4,7}]heptadec-13-en-4-yl acetate |
|---|
| Traditional Name | (1S,2S,3R,4S,7R,9S,10S,12R,15S)-1,2,9,12,15-pentahydroxy-10,14,17,17-tetramethyl-11-oxo-6-oxatetracyclo[11.3.1.0^{3,10}.0^{4,7}]heptadec-13-en-4-yl acetate |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H][C@@]12C[C@]([H])(O)[C@@]3(C)C(=O)[C@]([H])(O)C4=C(C)[C@@]([H])(O)C[C@@](O)([C@@]([H])(O)[C@]3([H])[C@@]1(CO2)OC(C)=O)C4(C)C |
|---|
| InChI Identifier | InChI=1S/C22H32O9/c1-9-11(24)7-22(29)18(28)16-20(5,17(27)15(26)14(9)19(22,3)4)12(25)6-13-21(16,8-30-13)31-10(2)23/h11-13,15-16,18,24-26,28-29H,6-8H2,1-5H3/t11-,12-,13+,15+,16-,18-,20+,21-,22+/m0/s1 |
|---|
| InChI Key | LHXBWTCSJBQSGI-QOBCYHTASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as taxanes and derivatives. These are diterpenoids with a structure based either on the taxane skeleton, or a derivative thereof. In term of phytochemistry, several derivatives of the taxane skeleton exist: 2(3->20)-abeotaxane, 3,11-cyclotaxane, 11(15->1),11(10->9)-abeotaxane, 3,8-seco-taxane, and 11(15->1)-abeotaxane, among others. More complex skeletons have been found recently, which include the taxane-derived [3.3.3] propellane ring system. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Diterpenoids |
|---|
| Direct Parent | Taxanes and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Taxane diterpenoid
- Cyclic alcohol
- Tertiary alcohol
- Carboxylic acid ester
- Ketone
- Oxetane
- Secondary alcohol
- Carboxylic acid derivative
- Polyol
- Monocarboxylic acid or derivatives
- Ether
- Dialkyl ether
- Organoheterocyclic compound
- Oxacycle
- Carbonyl group
- Organooxygen compound
- Hydrocarbon derivative
- Alcohol
- Organic oxide
- Organic oxygen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05fr-0002900000-3e2321f05092917278ff | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ac0-0004900000-62fb28bd6793f27d08b6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-06si-0339200000-f9353b267df037b55402 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0079-1003900000-f38ef7ad463b87cde60a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0adi-6109600000-8646c7e1d62746af4310 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9803000000-9e45730305b8671df756 | Spectrum |
|
|---|