| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-08-01 08:04:03 UTC |
|---|
| Update Date | 2016-08-01 19:18:31 UTC |
|---|
| Lmdb | LMDB00888 |
|---|
| Secondary Accession Numbers | None |
|---|
| Metabolite Identification |
|---|
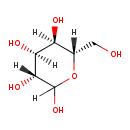
| Common Name | L-Altrose |
|---|
| Description | (3R,4S,5R,6S)-6-(hydroxymethyl)oxane-2,3,4,5-tetrol belongs to the class of organic compounds known as hexoses. These are monosaccharides in which the sugar unit is a is a six-carbon containing moeity (3R,4S,5R,6S)-6-(hydroxymethyl)oxane-2,3,4,5-tetrol is an extremely weak basic (essentially neutral) compound (based on its pKa). |
|---|
| Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C6H12O6 |
|---|
| Average Molecular Weight | 180.156 |
|---|
| Monoisotopic Molecular Weight | 180.063388106 |
|---|
| IUPAC Name | (3R,4S,5R,6S)-6-(hydroxymethyl)oxane-2,3,4,5-tetrol |
|---|
| Traditional Name | (3R,4S,5R,6S)-6-(hydroxymethyl)oxane-2,3,4,5-tetrol |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H]C1(O)O[C@@]([H])(CO)[C@]([H])(O)[C@]([H])(O)[C@@]1([H])O |
|---|
| InChI Identifier | InChI=1S/C6H12O6/c7-1-2-3(8)4(9)5(10)6(11)12-2/h2-11H,1H2/t2-,3-,4-,5+,6?/m0/s1 |
|---|
| InChI Key | WQZGKKKJIJFFOK-VSOAQEOCSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as hexoses. These are monosaccharides in which the sugar unit is a is a six-carbon containing moeity. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Hexoses |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hexose monosaccharide
- Oxane
- Secondary alcohol
- Hemiacetal
- 1,2-diol
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Hydrocarbon derivative
- Primary alcohol
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01q9-0900000000-865ff3c21e042be41194 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03ea-1900000000-ff4a32f071e4786201c6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0007-9200000000-417340d4949b8c0d2e3d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0900000000-9596d144f8fb2fdb5331 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01t9-4900000000-f0f8f46dba221853007d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-d0de6cdc5e0fb72d5f69 | Spectrum |
|
|---|