| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-08-01 22:02:19 UTC |
|---|
| Update Date | 2016-08-02 20:40:54 UTC |
|---|
| Lmdb | LMDB00928 |
|---|
| Secondary Accession Numbers | None |
|---|
| Metabolite Identification |
|---|
| Common Name | CE(16:0) |
|---|
| Description | CE(16:0) belongs to the family of cholesteryl esters, whose structure is characetized by a cholesterol esterified at the 3-position with a fatty acid. A cholesteryl ester is an ester of cholesterol. Fatty acid esters of cholesterol constitute about two-thirds of the cholesterol in the plasma. Cholesterol is a sterol (a combination steroid and alcohol) and a lipid found in the cell membranes of all body tissues, and transported in the blood plasma of all animals. The accumulation of cholesterol esters in the arterial intima (the innermost layer of an artery, in direct contact with the flowing blood) is a characteristic feature of atherosclerosis. Atherosclerosis is a disease affecting arterial blood vessels. It is a chronic inflammatory response in the walls of arteries, in large part to the deposition of lipoproteins (plasma proteins that carry cholesterol and triglycerides). CE(16:0) may also accumulate in hereditary hypercholesterolemia, an inborn error of metabolism. |
|---|
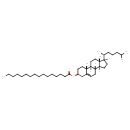
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (3beta)-Cholest-5-en-3-ol hexadecanoate | ChEBI | | 16:0 Cholesterol ester | ChEBI | | CE | ChEBI | | Cholesterol palmitate | ChEBI | | Cholesteryl hexadecanoate | ChEBI | | Hexadecanoic acid, cholesteryl ester | ChEBI | | Palmitic acid cholesteryl ester | ChEBI | | (3b)-Cholest-5-en-3-ol hexadecanoate | Generator | | (3b)-Cholest-5-en-3-ol hexadecanoic acid | Generator | | (3beta)-Cholest-5-en-3-ol hexadecanoic acid | Generator | | (3Β)-cholest-5-en-3-ol hexadecanoate | Generator | | (3Β)-cholest-5-en-3-ol hexadecanoic acid | Generator | | Cholesterol palmitic acid | Generator | | Cholesteryl hexadecanoic acid | Generator | | Hexadecanoate, cholesteryl ester | Generator | | Palmitate cholesteryl ester | Generator | | Cholesterol ester(16:0) | HMDB | | 1-Palmitoyl-cholesterol | HMDB | | Cholesteryl 1-hexadecanoic acid | HMDB | | Cholesterol ester(16:0/0:0) | HMDB | | Cholesterol 1-hexadecanoate | HMDB | | CE(16:0/0:0) | HMDB | | Cholesterol 1-hexadecanoic acid | HMDB | | Cholesterol 1-palmitoic acid | HMDB | | Cholesterol 1-palmitoate | HMDB | | Cholesteryl 1-palmitoate | HMDB | | Cholesteryl 1-hexadecanoate | HMDB | | 1-Hexadecanoyl-cholesterol | HMDB | | Cholesteryl 1-palmitoic acid | HMDB | | Cholest-5-en-3-yl palmitate | HMDB | | Cholest-5-ene-3-beta-yl palmitate | HMDB | | Cholesteryl palmitate | HMDB | | Cholesteryl palmitic acid | HMDB | | Hexadecanoate | HMDB | | Hexadecanoic acid | HMDB | | Hexadecanoic acid cholesteryl ester | HMDB | | 5-Cholesten-3beta-ol stearate | HMDB | | 5-Cholesten-3β-ol stearate | HMDB | | Cholest-5-en-3beta-ol stearate | HMDB | | Cholest-5-en-3β-ol stearate | HMDB | | Cholesterol hexadecanoate | HMDB | | CE(16:0) | Lipid Annotator |
|
|---|
| Chemical Formula | C43H76O2 |
|---|
| Average Molecular Weight | 625.0623 |
|---|
| Monoisotopic Molecular Weight | 624.584531676 |
|---|
| IUPAC Name | (1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-6-methylheptan-2-yl]tetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-7-en-5-yl hexadecanoate |
|---|
| Traditional Name | (1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-6-methylheptan-2-yl]tetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-7-en-5-yl hexadecanoate |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC=C4C[C@H](CC[C@]4(C)[C@@]3([H])CC[C@]12C)OC(=O)CCCCCCCCCCCCCCC)[C@H](C)CCCC(C)C |
|---|
| InChI Identifier | InChI=1S/C43H76O2/c1-7-8-9-10-11-12-13-14-15-16-17-18-19-23-41(44)45-36-28-30-42(5)35(32-36)24-25-37-39-27-26-38(34(4)22-20-21-33(2)3)43(39,6)31-29-40(37)42/h24,33-34,36-40H,7-23,25-32H2,1-6H3/t34-,36+,37+,38-,39+,40+,42+,43-/m1/s1 |
|---|
| InChI Key | BBJQPKLGPMQWBU-JADYGXMDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cholesteryl esters. Cholesteryl esters are compounds containing an esterified cholestane moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Steroid esters |
|---|
| Direct Parent | Cholesteryl esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cholesteryl ester
- Cholesterol
- Cholestane-skeleton
- Delta-5-steroid
- Carboxylic acid ester
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected and Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004r-1255039000-894288c747d8162be0b5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kr-4439030000-3c832b67193915b741ce | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ar1-4709120000-dc6542884db42b6750ad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00dr-0024009000-8a55f4904b3c9168d2af | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0049103000-e980336a44ede858695b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00ku-2029000000-106443473af958bc3183 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05r0-6135019000-47b0d4ec8524b1111db7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9220010000-56082adfd04894733c78 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9310100000-f9533a7c98f76ff25078 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0000009000-13d8ee384c55c248613b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0079-0091006000-a68e29dc6bde9a5fd623 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0abi-5951003000-df1f7ad38d6cbde09db4 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available | Spectrum |
|
|---|