| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-08-06 05:39:41 UTC |
|---|
| Update Date | 2016-08-06 20:10:56 UTC |
|---|
| Lmdb | LMDB01015 |
|---|
| Secondary Accession Numbers | None |
|---|
| Metabolite Identification |
|---|
| Common Name | Epitestosterone 17-glucuronide |
|---|
| Description | Testosterone glucuronide belongs to the class of organic compounds known as steroid glucuronide conjugates. These are sterol lipids containing a glucuronide moiety linked to the steroid skeleton. Thus, testosterone glucuronide is considered to be a steroid conjugate lipid molecule. Testosterone glucuronide is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. |
|---|
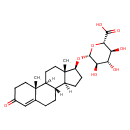
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Testosterone 17beta-(beta-D-glucuronide) | ChEBI | | Testosterone glucuronoside | ChEBI | | Testosterone 17b-(b-D-glucuronide) | Generator | | Testosterone 17β-(β-D-glucuronide) | Generator | | (17b)-3-Oxoandrost-4-en-17-yl b-D-glucopyranosiduronic acid | HMDB | | b-D-3-Oxoandrost-4-en-17b-yl glucopyranosiduronic acid | HMDB | | b-D-Androstane glucopyranosiduronic acid | HMDB | | Epitestosterone glucuronide | HMDB | | Testosterone 17-glucosiduronate | HMDB | | Testosterone 17-glucuronide | HMDB | | Testosterone glucopyranuronoside | HMDB | | Testosterone glucosiduronide | HMDB | | Testosterone glucuronate | HMDB | | Testosterone-glucuronide | HMDB | | (alpha)-Isomer OF testosterone glucuronate | HMDB |
|
|---|
| Chemical Formula | C25H36O8 |
|---|
| Average Molecular Weight | 464.5485 |
|---|
| Monoisotopic Molecular Weight | 464.241018128 |
|---|
| IUPAC Name | (2S,3S,4S,5R,6R)-6-{[(1S,2R,10R,11S,14S,15S)-2,15-dimethyl-5-oxotetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-6-en-14-yl]oxy}-3,4,5-trihydroxyoxane-2-carboxylic acid |
|---|
| Traditional Name | testosterone glucuronide |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H][C@@]12CC[C@H](O[C@@H]3O[C@@H]([C@@H](O)[C@H](O)[C@H]3O)C(O)=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C25H36O8/c1-24-9-7-13(26)11-12(24)3-4-14-15-5-6-17(25(15,2)10-8-16(14)24)32-23-20(29)18(27)19(28)21(33-23)22(30)31/h11,14-21,23,27-29H,3-10H2,1-2H3,(H,30,31)/t14-,15-,16-,17-,18-,19-,20+,21-,23+,24-,25-/m0/s1 |
|---|
| InChI Key | NIKZPECGCSUSBV-HMAFJQTKSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as steroid glucuronide conjugates. These are sterol lipids containing a glucuronide moiety linked to the steroid skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Steroidal glycosides |
|---|
| Direct Parent | Steroid glucuronide conjugates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Steroid-glucuronide-skeleton
- Androgen-skeleton
- Androstane-skeleton
- 3-oxo-delta-4-steroid
- 3-oxosteroid
- Oxosteroid
- Delta-4-steroid
- 1-o-glucuronide
- O-glucuronide
- Glucuronic acid or derivatives
- Hexose monosaccharide
- O-glycosyl compound
- Glycosyl compound
- Cyclohexenone
- Beta-hydroxy acid
- Monosaccharide
- Hydroxy acid
- Pyran
- Oxane
- Cyclic ketone
- Secondary alcohol
- Ketone
- Polyol
- Carboxylic acid
- Carboxylic acid derivative
- Organoheterocyclic compound
- Oxacycle
- Monocarboxylic acid or derivatives
- Acetal
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Alcohol
- Organooxygen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-002s-7374900000-df9fd1c0a23d7096cf26 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-014i-1606029000-ace2748138aab320b602 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00rj-0190600000-c12508c47c05623543ae | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0079-0390000000-6967a132771292eb3775 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00rl-0690000000-83b24c8c2b8d988ca1bf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03y0-1260900000-8a8bad65d00272674fb8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-1290200000-4ea1e726f18e48a97877 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-3190000000-b41d42aa036b4867d7fc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0000900000-bd3da6f548433d38332d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0571-9621300000-3022d77647816a41cad4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4r-9034100000-4cb79673d1b5b82c353c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0190500000-35f41b813389a2ebb839 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0301-0963500000-58248b539c8cea653977 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0cdl-1931000000-98e24f4d12e60e585a9c | Spectrum |
|
|---|