| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-08-06 05:39:48 UTC |
|---|
| Update Date | 2016-08-06 20:10:22 UTC |
|---|
| Lmdb | LMDB01017 |
|---|
| Secondary Accession Numbers | None |
|---|
| Metabolite Identification |
|---|
| Common Name | 1alpha,25-Dihydroxy-24-oxocholecalciferol |
|---|
| Description | (1S)-1,25-dihydroxy-24-oxocalciol, also known as 1,25-dihydroxy-24-oxo-vitamin D3 or 24-oxo-1a,25-dihydroxycholecalciferol, belongs to the class of organic compounds known as vitamin d and derivatives. Vitamin D and derivatives are compounds containing a secosteroid backbone, usually secoergostane or secocholestane. Thus, (1S)-1,25-dihydroxy-24-oxocalciol is considered to be a secosteroid lipid molecule (1S)-1,25-dihydroxy-24-oxocalciol is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. |
|---|
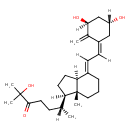
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,25-Dihydroxy-24-oxo-vitamin D3 | ChEBI | | 1,25-Dihydroxy-24-oxocholecalciferol | ChEBI | | 1alpha,25-Dihydroxy-24-oxocholecalciferol | ChEBI | | 1alpha,25-Dihydroxy-24-oxovitamin D3 | ChEBI | | 24-KDHVD3 | ChEBI | | 24-Keto-1,25-dihydroxyvitamin D3 | ChEBI | | 24-oxo-1alpha,25(OH)2D3 | ChEBI | | 24-oxo-1alpha,25-Dihydroxycholecalciferol | ChEBI | | 24-oxo-1alpha,25-Dihydroxyvitamin D3 | ChEBI | | 1a,25-Dihydroxy-24-oxocholecalciferol | Generator | | 1Α,25-dihydroxy-24-oxocholecalciferol | Generator | | 1a,25-Dihydroxy-24-oxovitamin D3 | Generator | | 1Α,25-dihydroxy-24-oxovitamin D3 | Generator | | 24-oxo-1a,25(OH)2D3 | Generator | | 24-oxo-1Α,25(OH)2D3 | Generator | | 24-oxo-1a,25-Dihydroxycholecalciferol | Generator | | 24-oxo-1Α,25-dihydroxycholecalciferol | Generator | | 24-oxo-1a,25-Dihydroxyvitamin D3 | Generator | | 24-oxo-1Α,25-dihydroxyvitamin D3 | Generator |
|
|---|
| Chemical Formula | C27H42O4 |
|---|
| Average Molecular Weight | 430.629 |
|---|
| Monoisotopic Molecular Weight | 430.308309832 |
|---|
| IUPAC Name | (6R)-6-[(1R,3aS,4E,7aR)-4-{2-[(1Z,3S,5R)-3,5-dihydroxy-2-methylidenecyclohexylidene]ethylidene}-7a-methyl-octahydro-1H-inden-1-yl]-2-hydroxy-2-methylheptan-3-one |
|---|
| Traditional Name | 24-kdhvd3 |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H]\C(\C(\[H])=C1/CCC[C@@]2(C)[C@@]1([H])CC[C@]2([H])[C@]([H])(C)CCC(=O)C(C)(C)O)=C1/C[C@@]([H])(O)C[C@]([H])(O)C1=C |
|---|
| InChI Identifier | InChI=1S/C27H42O4/c1-17(8-13-25(30)26(3,4)31)22-11-12-23-19(7-6-14-27(22,23)5)9-10-20-15-21(28)16-24(29)18(20)2/h9-10,17,21-24,28-29,31H,2,6-8,11-16H2,1,3-5H3/b19-9+,20-10-/t17-,21-,22-,23+,24+,27-/m1/s1 |
|---|
| InChI Key | BWFQMABKLLTETH-YGQRWWDYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as vitamin d and derivatives. Vitamin D and derivatives are compounds containing a secosteroid backbone, usually secoergostane or secocholestane. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Vitamin D and derivatives |
|---|
| Direct Parent | Vitamin D and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Triterpenoid
- Acyloin
- Tertiary alcohol
- Cyclic alcohol
- Alpha-hydroxy ketone
- Secondary alcohol
- Ketone
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03dj-0117900000-bdf5d96906157fc47f98 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03g1-3429200000-24be0d779e9279dd98de | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0771-4239100000-7af36c293dfb2e232d51 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0000900000-6bd7a1f1e9bdfad1fbd8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0w4i-0109500000-3584bd59c965e3de3dce | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0079-9107200000-d222490038642a3ed903 | Spectrum | | MS | Mass Spectrum (Electron Ionization) | Not Available | Spectrum |
|
|---|