| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-08-06 05:42:44 UTC |
|---|
| Update Date | 2016-08-06 20:10:57 UTC |
|---|
| Lmdb | LMDB01067 |
|---|
| Secondary Accession Numbers | None |
|---|
| Metabolite Identification |
|---|
| Common Name | Glucaric acid-1,4-lactone |
|---|
| Description | D-Glucaro-1,4-lactone, also known as D-saccharolactone or 1,4-D-glucarolactone, belongs to the class of organic compounds known as gamma butyrolactones. Gamma butyrolactones are compounds containing a gamma butyrolactone moiety, which consists of an aliphatic five-member ring with four carbon atoms, one oxygen atom, and bears a ketone group on the carbon adjacent to the oxygen atom. D-Glucaro-1,4-lactone has been detected, but not quantified in, several different foods, such as anatidaes (Anatidae), chickens (Gallus gallus), domestic pigs (Sus scrofa domestica), and milk (cow). This could make D-glucaro-1,4-lactone a potential biomarker for the consumption of these foods. Based on a literature review very few articles have been published on D-Glucaro-1,4-lactone. |
|---|
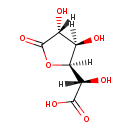
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,4-D-Glucarolactone | ChEBI | | 1,4-Glucarolactone | ChEBI | | D-Saccharolactone | ChEBI | | Saccharolactone | ChEBI | | Saccharolactone, monocalcium salt, (D)-isomer | HMDB | | Saccharolactone, (D)-isomer | HMDB | | Saccharolactone, monosodium salt, (D)-isomer | HMDB | | Glucarate-1,4-lactone | HMDB |
|
|---|
| Chemical Formula | C6H8O7 |
|---|
| Average Molecular Weight | 192.1235 |
|---|
| Monoisotopic Molecular Weight | 192.02700261 |
|---|
| IUPAC Name | (2S)-2-[(2S,3R,4R)-3,4-dihydroxy-5-oxooxolan-2-yl]-2-hydroxyacetic acid |
|---|
| Traditional Name | (S)-[(2S,3R,4R)-3,4-dihydroxy-5-oxooxolan-2-yl](hydroxy)acetic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H][C@@](O)(C(O)=O)[C@@]1([H])OC(=O)[C@]([H])(O)[C@@]1([H])O |
|---|
| InChI Identifier | InChI=1S/C6H8O7/c7-1-2(8)6(12)13-4(1)3(9)5(10)11/h1-4,7-9H,(H,10,11)/t1-,2-,3+,4+/m1/s1 |
|---|
| InChI Key | XECPAIJNBXCOBO-MMPJQOAZSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as gamma butyrolactones. Gamma butyrolactones are compounds containing a gamma butyrolactone moiety, which consists of an aliphatic five-member ring with four carbon atoms, one oxygen atom, and bears a ketone group on the carbon adjacent to the oxygen atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Lactones |
|---|
| Sub Class | Gamma butyrolactones |
|---|
| Direct Parent | Gamma butyrolactones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-hydroxy acid
- Dicarboxylic acid or derivatives
- Gamma butyrolactone
- Hydroxy acid
- Tetrahydrofuran
- Secondary alcohol
- Carboxylic acid ester
- Oxacycle
- Carboxylic acid
- Carboxylic acid derivative
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Organic oxygen compound
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (4 TMS) | splash10-014i-1971000000-6bc480f6132ff3b4f3a8 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-08i9-9300000000-3036b01985ecba9285d8 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-01di-6258900000-672b193020e251e41668 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004l-1900000000-2ec6e385ad66bb32dbf6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05r1-2900000000-8b979b3c9f43a65b913f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00du-9000000000-6d6b84a7185d89c6f912 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00tg-4900000000-21eb31d409626b01009c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-016s-2900000000-f8f32d204349bf201366 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0603-9300000000-96973bb25faba1a35f09 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0900000000-cc5bb18d1664b93a313a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00os-6900000000-1ba0094376fd3554d23c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ox-9000000000-ca8224ed70a4c6b3efbe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00bc-6900000000-b6467151416eacaa1e10 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-056r-9100000000-2c5aa67ce4b96aaedd10 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a6u-9000000000-fe8344a8a3a320b12c81 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|