| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-08-09 17:40:06 UTC |
|---|
| Update Date | 2016-08-09 21:33:46 UTC |
|---|
| Lmdb | LMDB01085 |
|---|
| Secondary Accession Numbers | None |
|---|
| Metabolite Identification |
|---|
| Common Name | Boldenone 17-O-b-D-glucuronide |
|---|
| Description | 6-{[(2R,14S,15S)-2,15-dimethyl-5-oxotetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-3,6-dien-14-yl]oxy}-3,4,5-trihydroxyoxane-2-carboxylic acid is an extremely weak basic (essentially neutral) compound (based on its pKa). |
|---|
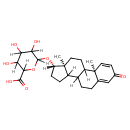
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 6-{[(2R,14S,15S)-2,15-dimethyl-5-oxotetracyclo[8.7.0.0,.0,]heptadeca-3,6-dien-14-yl]oxy}-3,4,5-trihydroxyoxane-2-carboxylate | Generator | | 6-{[(2R,14S,15S)-2,15-dimethyl-5-oxotetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-3,6-dien-14-yl]oxy}-3,4,5-trihydroxyoxane-2-carboxylate | Generator |
|
|---|
| Chemical Formula | C25H34O8 |
|---|
| Average Molecular Weight | 462.539 |
|---|
| Monoisotopic Molecular Weight | 462.225368055 |
|---|
| IUPAC Name | 6-{[(2R,14S,15S)-2,15-dimethyl-5-oxotetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-3,6-dien-14-yl]oxy}-3,4,5-trihydroxyoxane-2-carboxylic acid |
|---|
| Traditional Name | 6-{[(2R,14S,15S)-2,15-dimethyl-5-oxotetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-3,6-dien-14-yl]oxy}-3,4,5-trihydroxyoxane-2-carboxylic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H][C@@]1(CCC2([H])C3([H])CCC4=CC(=O)C=C[C@]4(C)C3([H])CC[C@]12C)OC1([H])OC([H])(C(O)=O)C([H])(O)C([H])(O)C1([H])O |
|---|
| InChI Identifier | InChI=1S/C25H34O8/c1-24-9-7-13(26)11-12(24)3-4-14-15-5-6-17(25(15,2)10-8-16(14)24)32-23-20(29)18(27)19(28)21(33-23)22(30)31/h7,9,11,14-21,23,27-29H,3-6,8,10H2,1-2H3,(H,30,31)/t14?,15?,16?,17-,18?,19?,20?,21?,23?,24-,25-/m0/s1 |
|---|
| InChI Key | WTOYIEKXMIILRQ-QQUUXXDRSA-N |
|---|
| Chemical Taxonomy |
|---|
| Classification | Not classified |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-029j-0090600000-687170b803011f915fea | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kr-0290000000-0da6269b23602ade4384 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kr-0390000000-86540924e7a32453d2d4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03y0-1160900000-164a02a4d7c11dd07910 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-1290200000-ba849673d0290575aaa1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-3190000000-1c6c902df5ed74d4ae39 | Spectrum |
|
|---|