| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-08-09 17:40:21 UTC |
|---|
| Update Date | 2016-08-09 21:34:19 UTC |
|---|
| Lmdb | LMDB01094 |
|---|
| Secondary Accession Numbers | None |
|---|
| Metabolite Identification |
|---|
| Common Name | Methyl-1-methyl-1,2,5,6-tetrahydro-3-pyridincarboxylat |
|---|
| Description | Arecoline, also known as arekolin or methylarecaiden, belongs to the class of organic compounds known as alkaloids and derivatives. These are naturally occurring chemical compounds that contain mostly basic nitrogen atoms. This group also includes some related compounds with neutral and even weakly acidic properties. Also some synthetic compounds of similar structure are attributed to alkaloids. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen, sulfur and more rarely other elements such as chlorine, bromine, and phosphorus. Arecoline is a secondary metabolite. Secondary metabolites are metabolically or physiologically non-essential metabolites that may serve a role as defense or signalling molecules. In some cases they are simply molecules that arise from the incomplete metabolism of other secondary metabolites. Based on a literature review a significant number of articles have been published on Arecoline. |
|---|
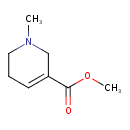
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Arecaidine methyl ester | ChEBI | | Arecaline | ChEBI | | Arecholine | ChEBI | | Arekolin | ChEBI | | Methylarecaiden | ChEBI | | Methylarecaidin | ChEBI | | 1,2,5,6-Tetrahydro-1-methylnicotinic acid, methyl ester | HMDB | | Arecholin | HMDB | | Arecolin | HMDB | | Arecoline base | HMDB | | Arecoline hydrobromide | HMDB | | Methyl 1,2,5, 6-tetrahydro-1-methylnicotinate | HMDB | | Methyl 1,2,5,6-tetrahydro-1-methylnicotinate | HMDB | | Methyl 1-methyl-1,2,5,6-tetrahydro-3-pyridinecarboxylate | HMDB | | Methyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate | HMDB | | Methyl 1-methyl-5,6-dihydro-2H-pyridine-3-carboxylate | HMDB | | Methyl N-methyl-1,2,5, 6-tetrahydronicotinate | HMDB | | Methyl N-methyl-1,2,5,6-tetrahydronicotinate | HMDB | | Methyl N-methyltetrahydronicotinate | HMDB | | N-Methyl-beta -carboxylic acid methyl ester | HMDB | | N-Methyltetrahydronicotinic acid, methyl ester | HMDB | | Nicotinic acid, 1,2,5,6-tetrahydro-1-methyl-, methyl ester | HMDB |
|
|---|
| Chemical Formula | C8H13NO2 |
|---|
| Average Molecular Weight | 155.1943 |
|---|
| Monoisotopic Molecular Weight | 155.094628665 |
|---|
| IUPAC Name | methyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate |
|---|
| Traditional Name | arecoline |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | COC(=O)C1=CCCN(C)C1 |
|---|
| InChI Identifier | InChI=1S/C8H13NO2/c1-9-5-3-4-7(6-9)8(10)11-2/h4H,3,5-6H2,1-2H3 |
|---|
| InChI Key | HJJPJSXJAXAIPN-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as alkaloids and derivatives. These are naturally occurring chemical compounds that contain mostly basic nitrogen atoms. This group also includes some related compounds with neutral and even weakly acidic properties. Also some synthetic compounds of similar structure are attributed to alkaloids. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen, sulfur and more rarely other elements such as chlorine, bromine, and phosphorus. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Not Available |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Alkaloids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alkaloid or derivatives
- Hydropyridine
- Methyl ester
- Enoate ester
- Alpha,beta-unsaturated carboxylic ester
- Amino acid or derivatives
- Carboxylic acid ester
- Tertiary amine
- Tertiary aliphatic amine
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organoheterocyclic compound
- Azacycle
- Organopnictogen compound
- Organic oxygen compound
- Carbonyl group
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Amine
- Organic oxide
- Hydrocarbon derivative
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-006w-9400000000-98b26a9d2867d710059e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0900000000-13bcb3825f7244e9bfd3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05fr-5900000000-4c54ca467a6647c61870 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00rw-9000000000-ff7785df405dc6c7b136 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0900000000-1de11420c61028c65308 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-1900000000-ff4ff3298d65bc8e1a2b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05fu-9300000000-f368ae9f151a5d63114c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-2900000000-938088a337b250c4c45d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fdk-7900000000-eacff4cd88f94b61cec0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a5c-9000000000-39cbbf9119989e64dd6e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-2900000000-9d51360c21b50a4980fc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05fs-8900000000-84101b2b84e59517def6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0007-9100000000-330ea72f81622bbe2ec1 | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0006-9400000000-c2ac70d939302e3f979b | Spectrum |
|
|---|